123663-49-0
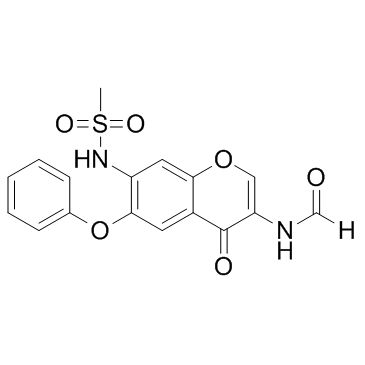
| Name | N-(7-(Methylsulfonamido)-4-oxo-6-phenoxy-4H-chromen-3-yl)formamide |
|---|---|
| Synonyms |
Iguratimod
N-(3-Formamido-4-oxo-6-phenoxy-4H-chromen-7-yl)methanesulfonamide N-[7-(Methanesulfonamido)-4-oxo-6-phenoxy-4H-chromen-3-yl]formamide N-[7-(methanesulfonamido)-4-oxo-6-phenoxychromen-3-yl]formamide |
| Description | Iguratimod is an antirheumatic agent, acts as an inhibitor of COX-2, with an IC50 of 20 μM (7.7 μg/mL), but shows no effect on COX-1. Iguratimod also inhibits macrophage migration inhibitory factor (MIF) with an IC50 of 6.81 μM. |
|---|---|
| Related Catalog | |
| Target |
COX-2:20 μM (IC50) MIF:6.81 μM (IC50) |
| In Vitro | Iguratimod (T-614) is an antirheumatic agent, acts as an inhibitor of COX-2, with an IC50 of 20 μM (7.7 μg/mL), but shows no effect on COX-1. Iguratimod (0.1, 1, 10 μg/mL) inhibits bradykinin-stimulated PGE2 release from fibroblasts. Iguratimod suppresses the COX activity from bradykinin stimulated fibroblasts in a concentration-dependent manner, with an IC50 of 48 μg/mL. Iguratimod (10 and 30 μg/mL) also dose-dependently inhibits COX-2 mRNA levels[1]. In addition, Iguratimod potently inhibits macrophage migration inhibitory factor (MIF) with an IC50 of 6.81 μM. Iguratimod is synergetic with glucocorticoids in vitro[3]. |
| In Vivo | Iguratimod (5 or 20 mg/kg) shows analgesic effect, significantly improves the pain withdrawal threshold of the left hind paw in dose-dependent manner in rats. Iguratimod (5 or 20 mg/kg) reduces the elevation of pERK1/2 and c-Fos in the spinal cord induced by cancer cell inoculation. Iguratimod also dose-dependently decreases the IL-6 levels in rats. In Iguratimod-treated rats, the activity of osteoclasts is weaker than the control group[2]. Iguratimod (20 mg/kg i.p.) shows significantly increased survival in BALB/c mice that are vulnerable to endotoxemia, and attenuates TNFα release measured in serum isolated 90 min post-LPS administration in wild-type C57BL/6 mice[3]. |
| Cell Assay | Briefly, human Raji B cells are plated at a density of 0.5 × 104 cells/well in a 96-well plate and synchronized by incubation for 24 h in RPMI 1640 medium supplemented with 0.1-0.5% FBS. Synchronized cells are pretreated with Iguratimod or vehicle for 30 min prior to stimulation with macrophage migration inhibitory factor (MIF) for 24 h. At 20 h BrdU is added to cells and quantified using a BrdU Cell proliferation assay kit[3]. |
| Animal Admin | Mice[3] Endotoxemia is induced by intraperitoneal injection of LPS from E. coli O111:B4. In BALB/c animals, 5 mg/kg LPS is used as a lethal dose for survival experiments; animals are treated with Iguratimod (20 mg/kg i.p.) 0.5 h prior to LPS, 6 h after LPS, and then once daily for 3 days and monitored for survival over 2 weeks. In C57BL/6 animals, 20 mg/kg LPS is used as non-lethal dose for plasma cytokine experiments; animals are pretreated with Iguratimod (20 mg/kg i.p.) twice, one dose each at 2 and 0.5 h prior to LPS administration, and euthanized at 90 min post-LPS by CO2 asphyxiation with cervical dislocation. Blood is collected by cardiac puncture and allowed to clot 20 min at room temperature and 20 min at 4°C; sera are isolated by centrifugation at 300 × g for 10 min and stored at −20°C for further analysis by TNFα ELISA (1:3 dilution)[3]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 580.6±60.0 °C at 760 mmHg |
| Melting Point | 238.0 to 242.0 °C |
| Molecular Formula | C17H14N2O6S |
| Molecular Weight | 374.368 |
| Flash Point | 304.9±32.9 °C |
| Exact Mass | 374.057251 |
| PSA | 123.09000 |
| LogP | 1.83 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.674 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2935009090 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |