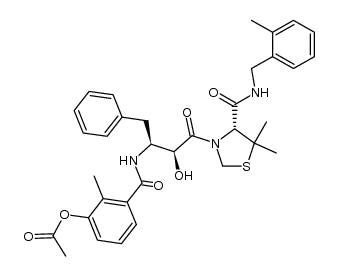
186538-00-1
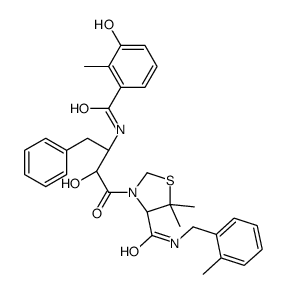
| Name | (4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylbutanoyl]-5,5-dimethyl-N-[(2-methylphenyl)methyl]-1,3-thiazolidine-4-carboxamide |
|---|---|
| Synonyms |
1kzk
AG1776 1msm 1msn 2anl JE-2147 JE2 KNI-764 |
| Description | JE-2147 (AG1776) is a potent dipeptide protease inhibitor with a Ki of 0.33 nM for HIV-1 protease. JE-2147 has effective activities against a wide spectrum of HIV-1, HIV-2, simian immunodeficiency virus, and various clinical HIV-1 strains in vitro[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 44 nM [HIV-1LAI (SI)], 24 nM [HIV-1Ba-L (SI)], 35 nM [HIV-1LAI (NSI)], 47 nM [HIV-2EHO (SI)][1] Ki:0.33 nM (HIV-1 protease)[2] |
| In Vitro | JE-2147 (0.01-0.05 μM; 7 days; PBMC and MT-2 cells) exhibits highly potent antiviral activity against HIV-1 with IC50s of 44 nM, 24 nM, 35 nM and 47 nM for HIV-1LAI (SI), HIV-1Ba-L (SI), HIV-1LAI (NSI) and HIV-2EHO (SI), respectively[1]. JE-2147 is effective against the replication of HIV-1 IIIB in various cells (T cell, B cell, macrophage, and PBMC) with IC50 values ranging from 31 to 160 nM and also had antiviral activity against simian immunodefidiency virus and HIV-2[2]. |
| In Vivo | JE-2147 exhibits good oral bioavailability and plasma pharmacokinetic profiles in dogs and rats[2]. Pharmacokinetic Parameters of JE-2147 in beagle dogs and male Sprague-Dawley rats[2]. Sprague-Dawley rats Beagle dogs i.v., 10 mg/kg i.d., 10 mg/kg i.v., 25 mg/kg p.o.(fed), 25 mg/kg p.o.(fasted), 25 mg/kg t1/2β (min) 93 / 94 / / Cmax (μM) / 0.70 ± 0.20 / 4.02 ± 0.72 5.30 ± 1.13 Tmax (min) / 60 / 90 60 F (%) / 41.6 ± 10.7 / 32.6 ± 0.06 37.1 ± 0.08 CL (L/h/kg) / / 0.88 ± 0.09 / / Vd, ss (L/kg) / / 1.58 / / AUC0-24 (μM·min) / / / 1009 1149 Animal Model: Beagle dogs and male Sprague-Dawley rats[2] Dosage: 25 mg/kg for dogs; 10 mg/kg for rats Administration: i.v. and i.d. for rats; i.v. and p.o. for dogs. Result: Exhibited favorable pharmacokinetic properties. |
| Density | 1.258g/cm3 |
|---|---|
| Boiling Point | 861.5ºC at 760mmHg |
| Molecular Formula | C32H37N3O5S |
| Molecular Weight | 575.71800 |
| Flash Point | 474.8ºC |
| Exact Mass | 575.24500 |
| PSA | 151.25000 |
| LogP | 5.06050 |
| Vapour Pressure | 1.2E-31mmHg at 25°C |
| Index of Refraction | 1.623 |