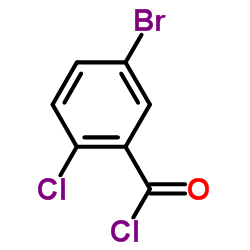
461432-26-8
| Name | dapagliflozin |
|---|---|
| Synonyms |
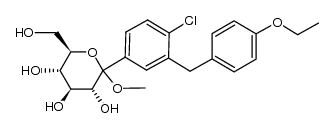
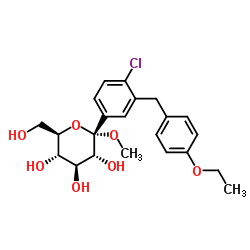
D-Glucitol,1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-,(1S)
Dapagliflozin Forxiga (1S)-1,5-Anhydro-1-[4-chloro-3-(4-ethoxybenzyl)phenyl]-D-glucitol Forxiga (TN) [14C]-Dapagliflozin (2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol dapaglifozin (2S,3R,4R,5S,6R)-2-[4-chloro-3-(4-ethoxybenzyl)phenyl]-6-(hydroxymethy)tetrahydro-2H-pyran-3,4,5-triol Farxiga S1548_Selleck (2S,3R,4R,5S,6R)-2-[4-chloro-3-(4-ethoxybenzyl)phenyl]-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol BMS 512148 |
| Description | Dapagliflozin (BMS-512148) is a sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. |
|---|---|
| Related Catalog | |
| Target |
SGLT2[1] |
| In Vitro | Dapagliflozin pretreatment of hypoxic HK2 cells significantly improves the cell viability in a dose-dependent manner. Dapagliflozin decreases Bax expression, the Bax/Bcl2 ratio, and PARP expression in hypoxic HK2 cells[2]. |
| In Vivo | At 11 mM glucose, dapagliflozin raises glucagon release from 18% to 32% of control, while the effect of dapagliflozin addition is minor at 1 mM glucose. At the intermediate glucose concentration of 6 mM, glucagon secretion is estimated to be 24% and 30% of control in the absence or presence of dapagliflozin, respectively[1]. Dapagliflozin pretreatment significantly reduces the number of TUNEL-positive cells in IR-injured kidneys. Dapagliflozin pretreatment significantly elevates the HIF1 expression in IR-injured renal tubular cells from mice[2]. Dapagliflozin (10 mg/kg, o.p.) causes a marked increase in urinary glucose in SGLT2i-mice. Dapagliflozin acutely suppresses BAT thermogenesis by reducing sympathetic nerve activity. Dapagliflozin enhances hepatic gluconeogenesis and glycogenolysis[3]. |
| Cell Assay | To perform the cell survival assay, cells are collected after 24 h incubation with vehicle or dapagliflozin pretreatment in 30-min ischemia and surviving cells are counted with Trypan blue staining. The percentage survival is determined by quantization of the relative viable number of treated cells divided by the viable number of untreated cells. |
| Animal Admin | 10-week-old male C57BL/6 mice weighing 30-33 g each are divided into five groups: vehicle (Vh)-treated sham (n=5), dapagliflozin-treated sham (n=5), Vh-treated IR (n=7), dapagliflozin-treated IR (n=7), and albendazole and dapagliflozin treated IR (n=7). Dapagliflozin is administrated via oral gavage at a dose of 10 mg/kg/day for 2 days, starting 24 h before surgery. Albendazole is injected subcutaneously 1 h before IR surgery. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 609.0±55.0 °C at 760 mmHg |
| Molecular Formula | C21H25ClO6 |
| Molecular Weight | 408.873 |
| Flash Point | 322.1±31.5 °C |
| Exact Mass | 408.133972 |
| PSA | 99.38000 |
| LogP | 4.42 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.614 |
|
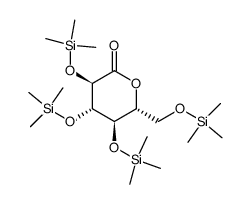
~43% 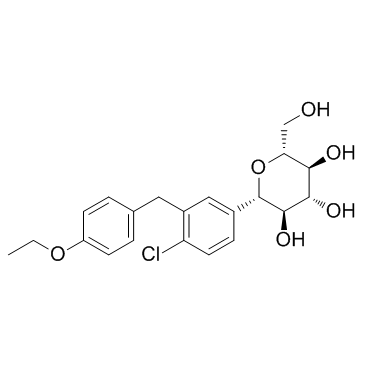
461432-26-8 |
| Literature: WO2010/48358 A2, ; Page/Page column 32-33 ; WO 2010/048358 A2 |
|
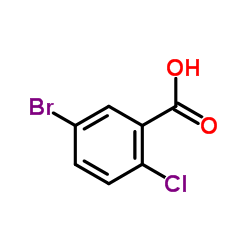
~% 
461432-26-8 |
| Literature: US2008/4336 A1, ; Page/Page column 21-22 ; |
|
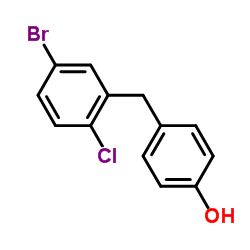
~% 
461432-26-8 |
| Literature: WO2013/152476 A1, ; |
|
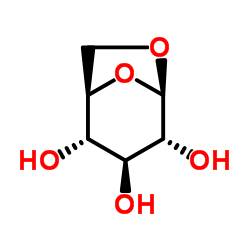
~% 
461432-26-8 |
| Literature: WO2013/152476 A1, ; |
|
~% 
461432-26-8 |
| Literature: WO2013/152476 A1, ; |
|
~% 
461432-26-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 4 p. 1236 - 1251 |
|
~% 
461432-26-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 4 p. 1236 - 1251 |
|
~% 
461432-26-8 |
| Literature: US2014/128595 A1, ; |
|
~% 
461432-26-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 4 p. 1236 - 1251 |