161172-51-6
| Name | Etalocib |
|---|---|
| Synonyms |
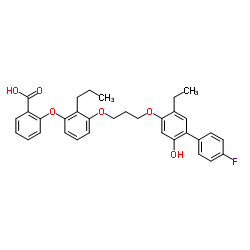
2-(3-{3-[(5-Ethyl-4'-fluoro-2-hydroxy-4-biphenylyl)oxy]propoxy}-2-propylphenoxy)benzoic acid
LY293111 2-(3-{3-[(5-ethyl-4'-fluoro-2-hydroxybiphenyl-4-yl)oxy]propoxy}-2-propylphenoxy)benzoic acid Etalocib LY-193111 |
| Description | Etalocib (LY293111), an orally active leukotriene B4 receptor antagonist, inhibits the binding of [3H]LTB4, with a Ki of 25 nM. Etalocib (LY293111) prevents LTB4-induced calcium mobilization with an lC50 of 20 nM. Etalocib (LY293111) induces apoptosis[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Etalocib (LY293111) elicits a concentration-dependent inhibition of LTB4 induced CD11b up-regulation[1]. Etalocib (LY293111) is an extremely potent and selective antagonist of human neutrophil function in vitro[2]. Etalocib (LY293111, 250 and 500 nM, 24-72 h) induces apoptosis and inhibits proliferation in human pancreatic cancer cells[3]. Cell Proliferation Assay[3] Cell Line: MiaPaCa-2 and AsPC-1 human pancreatic cancer cells.[3] Concentration: 500 nM. Incubation Time: 24, 48, and 72 h. Result: Caused both a concentration-dependent and time-dependent inhibition of thymidine incorporation in both MiaPaCa-2 and AsPC-1 human pancreatic cancer cells. Apoptosis Analysis[3] Cell Line: MiaPaCa-2 and AsPC-1 human pancreatic cancer cells. Concentration: 250 and 500 nM. Incubation Time: 24 h. Result: Induced apoptosis in human pancreatic cancer cells. |
| In Vivo | Etalocib (LY293111) produces a dose-related inhibition of acute leukotriene B4-induced airway obstruction when administered i.v. (ED50=14 µg/kg) or p.o. (ED50=0.4 mg/kg)[2]. Etalocib (LY293111, 10 mg/kg) inhibits A23187-induced lung inflammatory changes at 1 h[2]. Etalocib (LY293111, 250 mg/kg/day, orally) inhibits growth of human pancreatic cancer xenografts in athymic mice[3]. Animal Model: Guinea pigs[2]. Dosage: 1-10 mg/kg. Administration: Orally once. Result: A single 1 mg/kg oral dose inhibited excised lung gas volume increases by 76.7±7.1% (n=4, P<0.002) when given 8 h prior to leukotriene B4 challenge, and 28.6±20.3% (n=4, NS) when given 24 h before challenge. Had no effect (10 mg/kg) on pulmonary gas trapping at 1 h or 2 h after A23187 challenge. However, at 4 h, the pulmonary gas trapping response was significantly less than that of vehicle-treated controls and not different from sham values. The 10 mg/kg dose inhibited A23187-induced lung inflammatory changes at 1 h, but was without effect at 2 h or 4 h after challenge. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 656.8±55.0 °C at 760 mmHg |
| Molecular Formula | C33H33FO6 |
| Molecular Weight | 544.610 |
| Flash Point | 351.0±31.5 °C |
| Exact Mass | 544.226135 |
| PSA | 85.22000 |
| LogP | 7.61 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.594 |
| Storage condition | -20°C |
| Hazard Codes | Xi |
|---|