73121-56-9
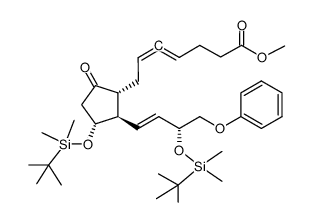
| Name | methyl 7-[(1R,2R,3R)-3-hydroxy-2-[(E,3R)-3-hydroxy-4-phenoxybut-1-enyl]-5-oxocyclopentyl]hepta-4,5-dienoate |
|---|---|
| Synonyms |
Enprostil (JAN/USAN/INN)
4,5-Heptadienoic acid, 7-[(1R,2R,3R)-3-hydroxy-2-[(1E,3R)-3-hydroxy-4-phenoxy-1-buten-1-yl]-5-oxocyclopentyl]-, methyl ester Syngard Enprostil (dl)-9-Keto-11a,15a-dihydroxy-16-phenoxy-17,18,19,20-tetranorprosta-4,5,13-trans-trienoic Acid Methyl Ester Camleed (TN) methyl (4,5,6S)-7-<(1R,2R,3R)-3-hydroxy-2-<(E)-(3R)-3-hydroxy-4-phenoxy-1-butenyl>-5-oxocyclopentyl>-4,5-heptadienoate Gardrine Methyl 7-{(1R,2R,3R)-3-hydroxy-2-[(1E,3R)-3-hydroxy-4-phenoxy-1-buten-1-yl]-5-oxocyclopentyl}-4,5-heptadienoate [1a,2b(1E,3R*),3a]-7-[3-Hydroxy-2-(3-hydroxy-4-phenoxy-1-butenyl)-5-oxocyclopentyl]-4,5-heptadienoic Acid Methyl Ester methyl (4,5,6R)-7-<(1R,2R,3R)-3-hydroxy-2-<(E)-(3R)-3-hydroxy-4-phenoxy-1-butenyl>-5-oxocyclopentyl>-4,5-heptadienoate Camleed Gardrin Methyl 7-{(1R,2R,3R)-3-hydroxy-2-[(1E,3R)-3-hydroxy-4-phenoxybut-1-en-1-yl]-5-oxocyclopentyl}hepta-4,5-dienoate |
| Description | Enprostil (RS 84135) is a prostaglandin E2 derivative. Enprostil can inhibit amogastrin-stimulated gastric acid secretion, as well as reducing the secretion of pepsin. Enprostil can also serve as an antiulcer agent, used for research of duodenal or gastric ulcers[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 552.9±50.0 °C at 760 mmHg |
| Molecular Formula | C23H28O6 |
| Molecular Weight | 400.46 |
| Flash Point | 185.7±23.6 °C |
| Exact Mass | 400.188599 |
| PSA | 93.06000 |
| LogP | 1.45 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.586 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
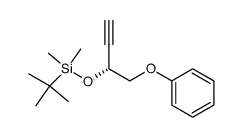
~67% 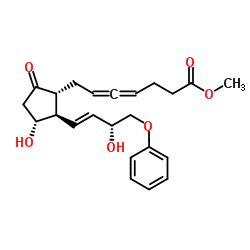
73121-56-9 |
| Literature: Gooding, Owen W.; Beard, Colin C.; Cooper, Gary F.; Jackson, David Y. Journal of Organic Chemistry, 1993 , vol. 58, # 14 p. 3681 - 3686 |
|
~% 
73121-56-9 |
| Literature: Synthesis, , # 8 p. 792 - 794 |
|
~% 
73121-56-9 |
| Literature: Synthesis, , # 8 p. 792 - 794 |
|
~% 
73121-56-9 |
| Literature: Synthesis, , # 8 p. 792 - 794 |
|
~% 
73121-56-9 |
| Literature: Journal of the Chemical Society, Chemical Communications, , # 10 p. 1251 - 1252 |
|
~% 
73121-56-9 |
| Literature: Journal of the Chemical Society, Chemical Communications, , # 10 p. 1251 - 1252 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |