1216459-54-9
| Name | 2-[4-(2-methylpropyl)phenyl]propanoic acid |
|---|---|
| Synonyms |
2-(4-Isobutylphenyl)propionic Acid-13C6
Ibuprofen-(ring-13C6) Ibuprofen-13C6 [13C6]-Ibuprofen |
| Description | Ibuprofen-13C6 ((±)-Ibuprofen-13C6) is a 13C labeled Ibuprofen (HY-78131). Ibuprofen ((±)-Ibuprofen) is a potent, orally active, selective COX-1 inhibitor with an IC50 value of 13 μM. Ibuprofen inhibits cell proliferation, angiogenesis, and induces cell apoptosis. Ibuprofen is a nonsteroidal anti-inflammatory agent and a nitric oxide (NO) donor. Ibuprofen ((±)-Ibuprofen) can be used in the research of pain, swelling, inflammation, infection, immunology, cancers[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| References |
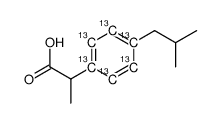
| Molecular Formula | C713C6H18O2 |
|---|---|
| Molecular Weight | 212.24 |
| Exact Mass | 212.15100 |
| PSA | 37.30000 |
| LogP | 3.07320 |