478296-72-9
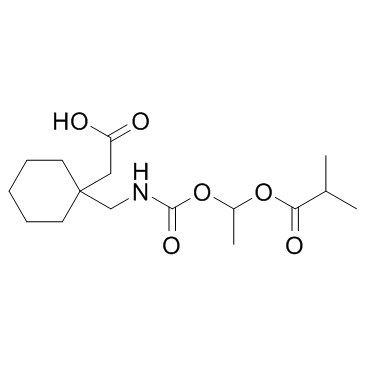
| Name | gabapentin enacarbil |
|---|---|
| Synonyms |
{[(1-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexaneacetic acid
{1-[({[1-(Isobutyryloxy)ethoxy]carbonyl}amino)methyl]cyclohexyl}acetic acid Solzira UNII-75OCL1SPBQ UNII:75OCL1SPBQ Horizant 2-[1-[[1-(2-methylpropanoyloxy)ethoxycarbonylamino]methyl]cyclohexyl]acetic acid ASP-8825 Gabapentin enacarbil Xenoport |
| Description | Gabapentin enacarbil (XP-13512) is a prodrug for the anticonvulsant and analgesic drug gabapentin.IC50 Value: Target: Calcium ChannelGabapentin enacarbil is an actively transported prodrug of gabapentin that provides sustained dose-proportional exposure to gabapentin and predictable bioavailability.in vitro: The prodrug (XP-13512) demonstrated active apical to basolateral transport across Caco-2 cell monolayers and pH-dependent passive permeability across artificial membranes. XP13512 inhibited uptake of (14)C-lactate by human embryonic kidney cells expressing monocarboxylate transporter type-1, and direct uptake of prodrug by these cells was confirmed using liquid chromatography-tandem mass spectrometry. XP13512 inhibited uptake of (3)H-biotin into Chinese hamster ovary cells overexpressing human sodium-dependent multivitamin transporter (SMVT) [1].in vivo: In 4 studies of healthy volunteers (136 subjects total), the pharmacokinetics of XP13512 immediate- and extended-release formulations were compared with those of oral gabapentin. XP13512 immediate-release (up to 2800 mg single dose and 2100 mg twice daily) was well absorbed (>68%, based on urinary recovery of gabapentin), converted rapidly to gabapentin, and provided dose-proportional exposure, whereas absorption of oral gabapentin declined with increasing doses to <27% at 1200 mg. Compared with 600 mg gabapentin, an equimolar XP13512 extended-release dose provided extended gabapentin exposure (time to maximum concentration, 8.4 vs 2.7 hours) and superior bioavailability (74.5% vs 36.6%) [2].Toxicity: Gabapentin's most common side effects in adult patients include dizziness, fatigue, weight gain, drowsiness, and peripheral edema (swelling of extremities). |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 482.0±20.0 °C at 760 mmHg |
| Melting Point | 65ºC |
| Molecular Formula | C16H27NO6 |
| Molecular Weight | 329.389 |
| Flash Point | 245.3±21.8 °C |
| Exact Mass | 329.183838 |
| PSA | 101.93000 |
| LogP | 3.07 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.481 |
| Storage condition | 2-8℃ |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
![Propanoic acid, 2-Methyl-, 1-[[[(2,5-dioxo-1-pyrrolidinyl)oxy]carbonyl]oxy]ethyl ester structure](https://image.chemsrc.com/caspic/232/860035-10-5.png)
![1-[(chlorocarbonyl)oxy]ethyl 2-methylpropionate structure](https://image.chemsrc.com/caspic/312/1164116-60-2.png)
![1-{[(α-chloroethoxy)carbonyl]aminomethyl}-1-cyclohexane acetic acid structure](https://image.chemsrc.com/caspic/356/850479-14-0.png)