20243-59-8
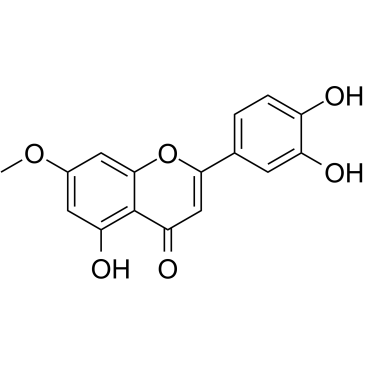
| Name | hydroxygenkwanin |
|---|---|
| Synonyms |
3',4',5-Trihydroxy-7-methoxyflavone
5,3',4'-trihydroxy-7-methoxyflavanone 7-O-Methylluteolin luteolin 7-methyl ester 3',4',5-trihydroxy-7-methoxyflavanone 5,3',4'-Trihydroxy-7-methoxyflavone 2-(3,4-Dihydroxyphenyl)-5-hydroxy-7-methoxy-4H-chromen-4-one Luteolin 7-methyl ether luteolin-7-methylether |
| Description | Hydroxygenkwanin (7-O-Methylluteolin), a natural flavonoid compound, is one of the main components of Lilac Daphne. Hydroxygenkwanin has anti-oxidant ability, anti-glioma ability and anticancer effect[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Hydroxygenkwanin (7-O-Methylluteolin) inhibits C6 glioma cell proliferation in a dose dependent manner[1]. Hydroxygenkwanin induces the cell cycle arrest by flow cytometry and inhibits colony formation ability and cell movement. Hydroxygenkwanin induces cell cycle arrest through p21 activation and causes intrinsic cell apoptosis pathway[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 602.7±55.0 °C at 760 mmHg |
| Melting Point | 225-227ºC |
| Molecular Formula | C16H12O6 |
| Molecular Weight | 300.263 |
| Flash Point | 230.6±25.0 °C |
| Exact Mass | 300.063385 |
| PSA | 100.13000 |
| LogP | 2.65 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.697 |
| HS Code | 2932999099 |
|---|
|
~% 
20243-59-8 |
| Literature: Ellemose, Steen; Kure, Niels; Torsell, Kurt B. G. Acta Chemica Scandinavica, 1995 , vol. 49, # 7 p. 524 - 529 |
|
~% 
20243-59-8 |
| Literature: Pankajamani; Seshadri Journal of the Indian Chemical Society, 1954 , vol. 31, p. 565 |
|
~0%
Detail
|
| Literature: Geiger, Hans; Casteele, Karel Vande; Sumere, Christiaan F. Van Zeitschrift fuer Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, 1984 , vol. 39, # 3 p. 393 - 396 |
|
~0% 
20243-59-8
Detail
|
| Literature: Geiger, Hans; Casteele, Karel Vande; Sumere, Christiaan F. Van Zeitschrift fuer Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, 1984 , vol. 39, # 3 p. 393 - 396 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |