500-65-2
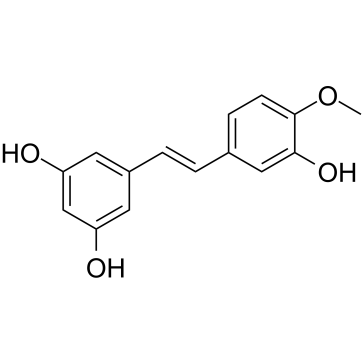
| Name | Rhapontigenin |
|---|---|
| Synonyms |
trans-1-(3,5-Dihydroxyphenyl)-2-(3-hydroxy-4-methoxyphenyl)ethylene
5-[(E)-2-(3-Hydroxy-4-methoxyphenyl)vinyl]-1,3-benzenediol rhapontigenin 5-[(E)-2-(3-Hydroxy-4-methoxyphenyl)vinyl]benzene-1,3-diol (E)-5-(3-Hydroxy-4-methoxystyryl)benzene-1,3-diol 3,3',5-Trihydroxy-4'-methoxy-trans-stilbene 5-[(E)-2-(3-hydroxy-4-methoxyphenyl)ethenyl]benzene-1,3-diol |
| Description | Rhapontigenin is a natural analog of resveratrol with anticancer, antioxidant, antifungal and antibacterial activities. Rhapontigenin is amechanism-based, potent and selective cytochrome P450 1A1 inactivator (IC50 = 400 nM). Rhapontigenin exhibits 400-fold and 23-fold selectivity for P450 1A1 over P450 1A2 and P450 1B1, respectively[1]. |
|---|---|
| Related Catalog | |
| Target |
CYP1A1:400 nM (IC50) |
| In Vitro | Rhapontigenin (0-250 μM; 24 hours) demonstrates concentration-dependent anti-cancer activity with an IC50 115μM in HEP G2 cells[1]. Rhapontigenin (20 μM; 20 hours) pre-treatment decreases TGF-β triggered increased snail expression in diverse cancer cells[2]. Rhapontigenin (0-20 μM; 6 hours) inhibits TGF-β-induced expression of N-cadherin, vimentin, and CA9 in a dose-dependent manner[2]. Rhapontigenin inhibits ADP- and collagen-induced platelet aggregation with IC50 values of 4 and 70 μg/ml, respectively[3]. Rhapontigenin demonstrates a strong inhibitory activity on the 13-hexosaminidase release induced by DNP-BSA, it exhibits IC50 value of 0.03 mM in RBL 2H3 cells[3]. Western Blot Analysis[2] Cell Line: HeLa, A549,769-P cells Concentration: 0 μM; 2.5 μM; 5 μM; 10 μM; 20 μM Incubation Time: 6 hours Result: Induced ubiquitination and degradation of HIF-1α. |
| In Vivo | Rhapontigenin (intraperitoneal injection; 25mg/kg) shows significant protection from death due to pulmonary thrombosis in mice, those samples are orally administered 90 min before tail vein injection of epinephrine and collagen[3]. Animal Model: ICR mice[3] Dosage: 25mg/kg Administration: 25mg/kg; intraperitoneal injection Result: Showed anti-thrombosis activity with 60% protection. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 503.6±38.0 °C at 760 mmHg |
| Melting Point | 186-187ºC |
| Molecular Formula | C15H14O4 |
| Molecular Weight | 258.269 |
| Flash Point | 258.4±26.8 °C |
| Exact Mass | 258.089203 |
| PSA | 69.92000 |
| LogP | 2.82 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.722 |