108657-10-9
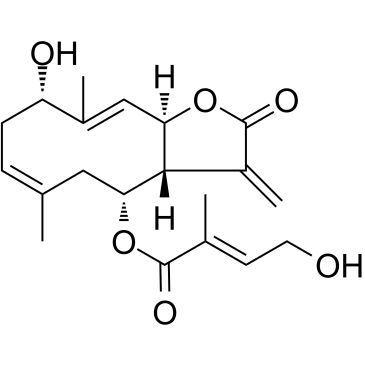
| Name | (1E,3β,4E,6α,7β,8β)-3-hydroxy-8-[(4-hydroxytigloyl)oxy]germacra-1(10),4,11(13)-trieno-12,6-lactone |
|---|---|
| Synonyms |
3β-hydroxy-8β-[4'-hydroxytigloyloxy]costunolide
(2E)-4-hydroxy-2-methylbut-2-enoic acid (3aR,4R,6E,9S,10E,11aR)-2,3,3a,4,5,8,9,11a-octahydro-9-hydroxy-6,10-dimethyl-3-methylene-2-oxocyclodeca[b]furan-4-yl ester (3aR,4S,6Z,9S,10Z,11aR)-9-Hydroxy-6,10-dimethyl-3-methylene-2-oxo-2,3,3a,4,5,8,9,11a-octahydrocyclodeca[b]furan-4-yl (2E)-4-hydroxy-2-methyl-2-butenoate eupalinolide K |
| Description | Eupalinolide K, a sesquiterpene lactones compound from Eupatorium lindleyanum, is a STAT3 inhibitor. Eupalinolide K is a Michael reaction acceptor (MRA) [1]. |
|---|---|
| Related Catalog | |
| Target |
STAT3 |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 574.4±50.0 °C at 760 mmHg |
| Molecular Formula | C20H26O6 |
| Molecular Weight | 362.417 |
| Flash Point | 201.5±23.6 °C |
| Exact Mass | 362.172943 |
| PSA | 93.06000 |
| LogP | 2.29 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.557 |