652-78-8
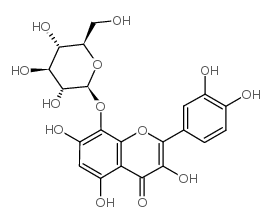
| Name | gossypin |
|---|---|
| Synonyms |
Gossypetin 8-O-glucoside
3,5,7,8,3',4'-hexahydroxyflavone-8-O-glucopyranoside 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-8-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one 3,3',4',5,7-pentahydroxyflavone-8-O-glucoside Gossypin Gossypetin-8-glucoside |
| Description | Gossypin is a flavone isolated from Hibiscus vitifolius and has antioxidant, antiinflammatory, anticancer, anticataract, antidiabetic, analgesic and hepatoprotective activities. Gossypin inhibits NF-κB and NF-κB-regulated gene expression. Gossypin inhibits RANKL-induced osteoclastogenesis both in mouse primary bone marrow cells and RAW 264.7 cells in vitro[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: NF-κB[1] |
| References |
| Density | 1.883 g/cm3 |
|---|---|
| Boiling Point | 886ºC at 760 mmHg |
| Melting Point | 229-230ºC |
| Molecular Formula | C21H20O13 |
| Molecular Weight | 480.37600 |
| Flash Point | 310.8ºC |
| Exact Mass | 480.09000 |
| PSA | 230.74000 |
| Index of Refraction | 1.799 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36-37 |
| RIDADR | NONH for all modes of transport |