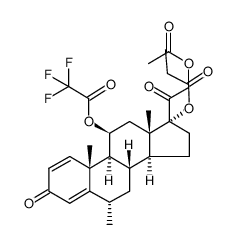
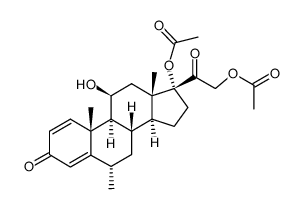
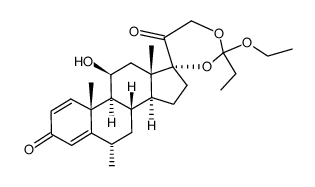
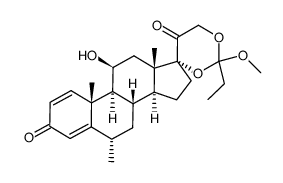
86401-95-8
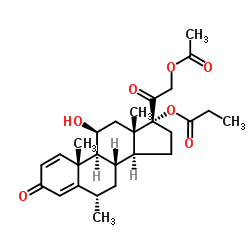
| Name | Methylprednisolone aceponate |
|---|---|
| Synonyms |
Aceponato de metilprednisolona [Spanish]
(6a,11b)-21-(Acetyloxy)-11-hydroxy-6-methyl-17-(1-oxopropoxy)pregna-1,4-diene-3,20-dione Advantan Methylprednisoloni aceponas [Latin] SH-440 Aceponate de methylprednisolone [French] Methylprednisolone aceponate [(6S,8S,9S,10R,11S,13S,14S,17R)-17-(2-acetyloxyacetyl)-11-hydroxy-6,10,13-trimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl] propanoate (6α,11β)-21-(acetyloxy)-11-hydroxy-6-methyl-3,20-dioxopregna-1,4-dien-17-yl propanoate (6α,11β)-21-Acetoxy-11-hydroxy-6-methyl-3,20-dioxopregna-1,4-dien-17-yl propionate 11b,17,21-Trihydroxy-6a-methylpregna-1,4-diene-3,20-dione 21-Acetate 17-Propionate |
| Description | Methylprednisolone aceponate (ZK 91588) is a glucocorticoid and anti-inflammatory agent with weak systemic effects. Methylprednisolone aceponate is a selective glucocorticoid receptor Ligand.Methylprednisolone aceponate can be used for research of eczema and other inflammatory skin disorders[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Methylprednisolone aceponate 抑制胶原酶启动子活性 (在 HeLa 细胞中)、LPS 诱导的 IL-12p40 分泌 (在人 PBMC 中) 和植物凝集素诱导的 IFN-γ 分泌 (在人 PBMC 中),IC50s 分别为 9.3 , 16.8, 15.2 nM[3]. Methylprednisolone aceponate 激活 MMTV 启动子和 TAT 活性,EC50 分别为 21.8 和 20.5 nM[3]。 |
| In Vivo | Methylprednisolone aceponate (局部应用, 50 μL, 巴豆油引起的伊文蓝水肿模型) 具有抗炎作用,IC50 为 0.0015%,全身副作用低[1]. Methylprednisolone aceponate (0.0001%-0.1%,局部应用) 在小鼠和大鼠的刺激性接触性皮炎中抑制水肿形成,ED50 为 0.002%[3]。 Animal Model: Irritant contact dermatitis mice and rat[3] Dosage: 0.0001%-0.1% Administration: Topically applied, 10 µL for mice and 20 µL for rats. Result: Significantly inhibited ear inflammation. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 595.8±50.0 °C at 760 mmHg |
| Molecular Formula | C27H36O7 |
| Molecular Weight | 472.570 |
| Flash Point | 193.1±23.6 °C |
| Exact Mass | 472.246094 |
| PSA | 106.97000 |
| LogP | 4.01 |
| Vapour Pressure | 0.0±3.8 mmHg at 25°C |
| Index of Refraction | 1.560 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
86401-95-8 |
| Literature: US4587236 A1, ; |
|
~% 
86401-95-8 |
| Literature: US4587236 A1, ; |
|
~% 
86401-95-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 33, # 5 p. 1889 - 1898 |
|
~% 
86401-95-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 33, # 5 p. 1889 - 1898 |
|
~% 
86401-95-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 33, # 5 p. 1889 - 1898 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |