125973-56-0
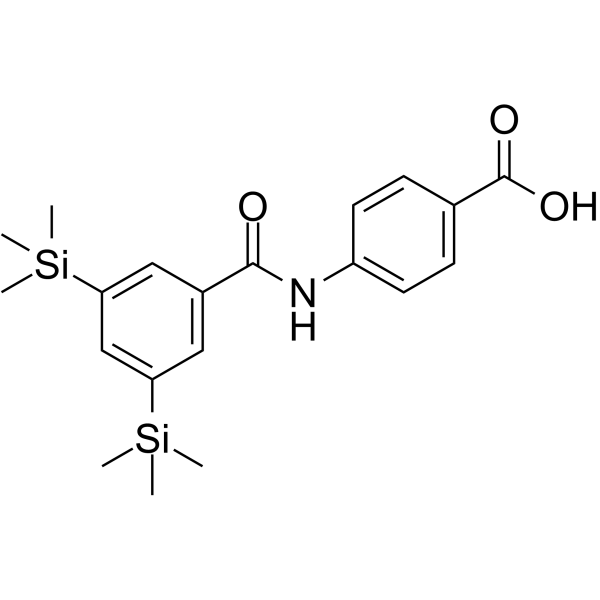
| Name | 4-[[3,5-bis(trimethylsilyl)benzoyl]amino]benzoic acid |
|---|---|
| Synonyms |
Am-555S
4-{[3,5-Bis(trimethylsilyl)benzoyl]amino}benzoic acid Amsilarotene UNII-Q1418F39MH TAC 101 |
| Description | Amsilarotene (TAC-101; Am 555S), an orally active synthetic retinoid, has selective affinity for retinoic acid receptor α (RAR-α) binding with Ki of 2.4, 400 nM for RAR-α and RAR-β. Amsilarotene induces the apoptotic of human gastric cancer, hepatocellular carcinoma and ovarian carcinoma cells. Amsilarotene can be used for the research of cancer[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
RARα:2.4 nM (Ki) RARβ:400 nM (Ki) |
| In Vitro | Amsilarotene (0, 10, 25 μM; 24 hours) induces apoptosis of human epithelial ovarian carcinoma-derived cell lines in a concentration-dependent manner[2]. Amsilarotene (10, 20 μM; 0, 3, 6, and 9 days) inhibits the proliferation of BxPC-3 and MIAPaCa-2 cells[3]. Amsilarotene (10 μM; 48 hours) increases the proportion of sensitive BxPC-3 cells in the G1 phase[3]. Amsilarotene (10 μM; 0, 3, 6, 24, 48, 72 hours) inhibits the retinoblastoma-gene product (RB) phosphorylation in BxPC-3 cells between 24 and 72 hours[3]. Apoptosis Analysis[2] Cell Line: RMG-I, RMG-II, RTSG, RMUG-S, RMUG-L, and KF cells Concentration: 0, 10, 25 μM Incubation Time: 24 hours Result: Induced apoptosis in a concentration-dependent manner in all of the cell lines, except KF cells. Cell Proliferation Assay[3] Cell Line: BxPC-3, MIAPaCa-2, AsPC-1 cells Concentration: 10 and 20 μM Incubation Time: 0, 3, 6, and 9 days. Result: Inhibited the proliferation of BxPC-3 and MIAPaCa-2 cells, but not the proliferation of AsPC-1 cells. Cell Cycle Analysis[3] Cell Line: Sensitive BxPC-3 cells Concentration: 10 μM Incubation Time: 48 hours Result: The proportion of cells in the G1 phase increased from 43% of untreated control cells to 86% |
| In Vivo | Amsilarotene (8 mg/kg/day orally for 30 days) inhibits the RMG-II tumor growth in nude mice[2]. Animal Model: 6-week-old female BALB/c nu/nu mice with subcutaneous RMG-II tumors[2] Dosage: 8 mg/kg/day Administration: Orally for 30 days Result: The maximal tumor growth-inhibiting effect was seen on day 31 of administration, when there was a 45% reduction of relative tumor volume (RTV). |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 403.3±45.0 °C at 760 mmHg |
| Molecular Formula | C20H27NO3Si2 |
| Molecular Weight | 385.604 |
| Flash Point | 197.7±28.7 °C |
| Exact Mass | 385.152954 |
| PSA | 66.40000 |
| LogP | 7.73 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.550 |
| Hazard Codes | Xi |
|---|
| Precursor 5 | |
|---|---|
| DownStream 0 | |