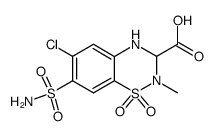
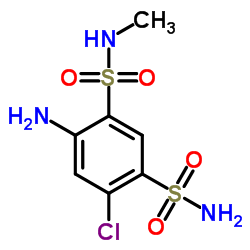
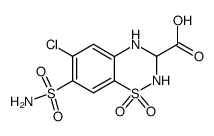
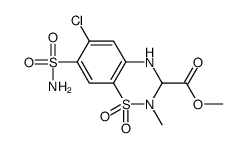
42583-55-1
| Name | Methyl 6-chloro-2-methyl-7-sulfamoyl-3,4-dihydro-2H-1,2,4-benzoth iadiazine-3-carboxylate 1,1-dioxide |
|---|---|
| Synonyms |
carmetizide
3.8-Dinitro-6-chlor-phenanthridin 6-chloro-3,8-dinitro-phenanthridine 6-Chlor-3,8-dinitro-phenanthridin 6-Chlor-3.4-dihydro-3-methoxycarbonyl-2-methyl-7-sulfamoyl-2H-1.2.4-benzothiadiazin-1.1-dioxid |
| Density | 1.618g/cm3 |
|---|---|
| Boiling Point | 589.4ºC at 760mmHg |
| Molecular Formula | C10H12ClN3O6S2 |
| Molecular Weight | 369.80200 |
| Flash Point | 310.3ºC |
| Exact Mass | 368.98600 |
| PSA | 152.63000 |
| LogP | 2.47030 |
| Index of Refraction | 1.605 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
42583-55-1 |
| Literature: Close,W.J. et al. Journal of Organic Chemistry, 1961 , vol. 26, p. 3423 - 3428 |
|
~% 
42583-55-1 |
| Literature: Close,W.J. et al. Journal of Organic Chemistry, 1961 , vol. 26, p. 3423 - 3428 |
|
~% 
42583-55-1 |
| Literature: Close,W.J. et al. Journal of Organic Chemistry, 1961 , vol. 26, p. 3423 - 3428 |
|
~% 
42583-55-1 |
| Literature: Close,W.J. et al. Journal of Organic Chemistry, 1961 , vol. 26, p. 3423 - 3428 |
|
~% 
42583-55-1 |
| Literature: Close,W.J. et al. Journal of Organic Chemistry, 1961 , vol. 26, p. 3423 - 3428 |
|
~% 
42583-55-1 |
| Literature: Close,W.J. et al. Journal of Organic Chemistry, 1961 , vol. 26, p. 3423 - 3428 |
|
~% 
42583-55-1 |
| Literature: Close,W.J. et al. Journal of Organic Chemistry, 1961 , vol. 26, p. 3423 - 3428 |