CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DF4936700
-
CAS REGISTRY NUMBER :
-
69866-21-3
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C37-H33-N7-O8
-
MOLECULAR WEIGHT :
-
703.77
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>8 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>8800 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
6900 ng/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Liver - other changes Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
9 ug/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Liver - other changes Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1 ug/kg
-
TOXIC EFFECTS :
-
Liver - fatty liver degeneration
-
TYPE OF TEST :
-
Micronucleus test
MUTATION DATA
-
TYPE OF TEST :
-
DNA damage
-
TEST SYSTEM :
-
Mammal - species unspecified Lymphocyte
-
DOSE/DURATION :
-
7400 nmol/L
-
REFERENCE :
-
CNREA8 Cancer Research. (Public Ledger Building, Suit 816, 6th & Chestnut Sts., Philadelphia, PA 19106) V.1- 1941- Volume(issue)/page/year: 42,999,1982 *** REVIEWS *** TOXICOLOGY REVIEW JANTAJ Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo, 141, Japan) V.2-5, 1948-52; V.21- 1968- Volume(issue)/page/year: 39,319,1986
|
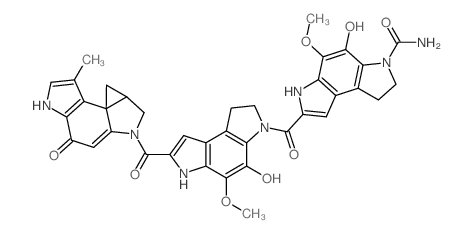
![(S)-7-(7-(1-(chloromethyl)-5-hydroxy-8-methyl-1,2,3,6-tetrahydropyrrolo[3,2-e]indole-3-carbonyl)-4-hydroxy-5-methoxy-1,2,3,6-tetrahydropyrrolo[3,2-e]indole-3-carbonyl)-4-hydroxy-5-methoxy-1,6-dihydropyrrolo[3,2-e]indole-3(2H)-carboxamide structure](https://image.chemsrc.com/caspic/010/112764-71-3.png)
![(S)-1,6-dihydro-1-(hydroxymethyl)-8-methyl-5-(phenylmethoxy)-benzo[1,2-b:4,3-b']dipyrrole-3(2H)-carboxylic acid 1,1-dimethylethyl ester structure](https://image.chemsrc.com/caspic/082/112836-67-6.png)
![(S)-1-(chloromethyl)-1,6-dihydro-8-methyl-5-(phenylmethoxy)-benzo[1,2-b:4,3-b']dipyrrole-3(2H)-carboxylic acid 1,1-dimethylethyl ester structure](https://image.chemsrc.com/caspic/243/110314-50-6.png)
![5-(benzyloxy)-1,2,3,6-tetrahydro-8-methylbenzo[1,2-b:4,3-b']dipyrrole-1-methanol structure](https://image.chemsrc.com/caspic/125/112243-83-1.png)
![(1RS)-3-(Benzenesulfonyl)-5-(benzyloxy)-1-(hydroxymethyl)-8-methyl-1,2-dihydro-3H-pyrrolo[3,2-e]indole structure](https://image.chemsrc.com/caspic/489/112764-67-7.png)
![6-benzoyl-5-(benzyloxy)-1,2-dihydro-8-methyl-1-methylidene-3-(phenylsulfonyl)-3H-pyrrolo[3,2-e]indole structure](https://image.chemsrc.com/caspic/367/112793-55-2.png)
![6-benzoyl-5-(benzyloxy)-1,2-dihydro-1-(hydroxymethyl)-8-methyl-3-(phenylsulfonyl)-3H-pyrrolo[3,2-e]indole structure](https://image.chemsrc.com/caspic/027/112764-66-6.png)
