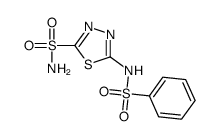
3368-13-6
| Name | 5-(benzenesulfonamido)-1,3,4-thiadiazole-2-sulfonamide |
|---|---|
| Synonyms |
3dbu
benzenesulfonylamino-[1,3,4]thiadiazole-2-sulfonic acid amide 5-phenylsulfonamide-1,3,4-thiadiazole-2-sulfonamide 5-[(phenylsulfonyl)amino]-1,3,4-thiadiazole-2-sulfonamide W 1803 2-(Phenylsulfonylamino)-1,3,4-thiadiazole-5-sulfonamide 5-benzenesulfonamido-1,3,4-thiadiazol-2-sulfonamide 1,3,4-Thiadiazole-2-sulfonamide,5-((phenylsulfonyl)amino) D8W 2-benzenesulfonamido-1,3,4-thiadiazole-5-sulfonamide Benzolamide (BZA) BENZOLAMIDE |
| Description | Benzolamide (CL11366) is a potent carbonic anhydrase (CA) inhibitor, with Kis of 15 nM, 9 nM, 94 nM and 78 nM for hCA I, hCA II, EcoCAγ and VchCAγ, respectively. Benzolamide also inhibits CAS3, with a Ki of 54 nM. Benzolamide can be used for the research of glaucoma and seizures[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 15 nM (hCA I), 9 nM (hCA II), 94 nM (EcoCAγ), 78 nM (VchCAγ), 54 nM (CAS3)[1][2] |
| In Vitro | Benzolamide inhibits hCA I, hCA II, EcoCAγ and VchCAγ, with Kis of 15 nM, 9 nM, 94 nM and 78 nM, respectively[1]. Benzolamide shows selectivity for CAS3 (Ki=54 nM) over CAS1 (Ki=2115 nM) and CAS2 (Ki=410 nM)[2]. |
| In Vivo | Benzolamide (90 µmol/kg; i.p.) decreases brain pH and suppresses electrographic post-asphyxia seizures in rats[3]. Animal Model: Male and female Wistar Han rats (11-day-old)[3] Dosage: 90 µmol/kg Administration: A single i.p. Result: Induced a fast brain acidosis of a comparable magnitude. Suppressed electrographic seizures after asphyxia by slowing down the recovery of brain pH. |
| References |
| Density | 1.746g/cm3 |
|---|---|
| Boiling Point | 585.9ºC at 760mmHg |
| Molecular Formula | C8H8N4O4S3 |
| Molecular Weight | 320.36900 |
| Flash Point | 308.2ºC |
| Exact Mass | 319.97100 |
| PSA | 177.11000 |
| LogP | 2.92120 |
| Index of Refraction | 1.689 |
| Storage condition | 2-8°C |
| HS Code | 2935009090 |
|---|
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |