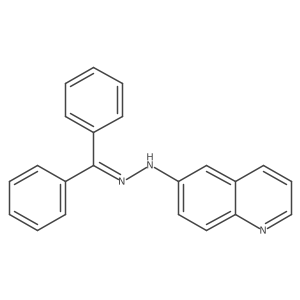
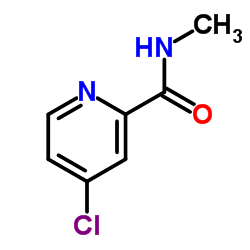
1020172-07-9
| Name | 4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide |
|---|---|
| Synonyms |
N-[3-tert-Butyl-1-(quinolin-6-yl)-1H-pyrazol-5-yl]-N'-[2-fluoro-4-[(2-(methylcarbamoyl)pyridin-4-yl)oxy]phenyl]urea
DCC2036 Rebastinib 4-[3-Fluoro-4-({[3-(2-methyl-2-propanyl)-1-(6-quinolinyl)-1H-pyrazol-5-yl]carbamoyl}amino)phenoxy]-N-methyl-2-pyridinecarboxamide 4-[4-({[3-Tert-Butyl-1-(Quinolin-6-Yl)-1h-Pyrazol-5-Yl]carbamoyl}amino)-3-Fluorophenoxy]-N-Methylpyridine-2-Carboxamide Rebastinib [USAN] 1-(3-tert-butyl-1-(quinolin-6-yl)-1H-pyrazol-5-yl)-3-(2-fluoro-4-(2-(methylcarbamoyl)pyridin-4-yloxy)phenyl)urea 4-(4-(3-(3-tert-butyl-1-(quinolin-6-yl)-1H-pyrazol-5-yl)ureido)-3-fluorophenoxy)-N-methylpicolinamide DP-1919 DCC-2036 |
| Description | Rebastinib (DCC-2036) is a conformational control Bcr-Abl inhibitor for Abl1WT and Abl1T315I with IC50 of 0.8 nM and 4 nM, also inhibits SRC, KDR, FLT3, and Tie-2, and low activity to seen towards c-Kit. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.75±0.11 nM (ABL1WT), 2±0.3 nM (FLT3), 4±0.3 nM (KDR), 6±0.3 nM (TIE2), 34±6 nM (SRC)[1] |
| In Vitro | Rebastinib (DCC-2036) inhibits ABL1native and the gatekeeper mutant ABL1T315I with IC50 of 0.8 nM and 4 nM, respectively. Rebastinib potently (IC50 0.82 nM) inhibits u-ABL1native, which is thought to exist predominantly in the inactive type II conformation. In addition, Rebastinib also strongly inhibits p-ABL1native (IC50 2 nM), which more readily adopts an active, Type I conformation. More significantly, Rebastinib potently inhibits both u-ABL1T315I (IC50 5 nM) and p-ABL1T315I (IC50 4 nM), both of which exist predominately in the Type I conformation due to stabilization of an activating hydrophobic spine by the T315I mutation. Rebastinib also potently inhibits ABL1H396P (IC50 1.4 nM), which, like ABL1T315I, is predisposed to exist predominately in a Type I activated conformation due to the restricted backbone torsional angles imposed by the mutant Pro396. In addition to ABL1, Rebastinib also inhibits the SRC family kinases LYN, SRC, FGR, and HCK, and PDGFRα, and PDGFRβ with IC50 of 29±1, 34±6, 38±1, 40±1, 70±10 and 113±10 nM, respectively. Notably, Rebastinib spared c-KIT (IC50 481 nM). Rebastinib effectively inhibits the proliferation of Ba/F3 cells expressing native BCR-ABL1native (IC50 5.4 nM). Rebastinib also inhibits proliferation of the Ph+ cell line K562 (IC50 5.5 nM). REBASTINIB (DCC-2036) also inhibits proliferation of several common TKI-resistant mutants of BCR-ABL1, including G250E, Q252H, Y235F, E255K, V299L, F317L, and M351T, at IC50s ranging from 6-150 nM. Rebastinib effectively inhibits autophosphorylation of BCR-ABL1native (IC50 29 nM) and BCR-ABL1T315I (IC50 18 nM), as well as the phosphorylation of STAT5 in both cell lines (IC50 28 nM and 13 nM, respectively)[1]. |
| In Vivo | A single oral dose of Rebastinib (DCC-2036) at 100 mg/kg afforded circulating plasma levels that exceeded 12 μM for up to 24 hours (data not shown), and effectively inhibited BCR-ABL1 signaling for up to 8 hours in Ba/F3-BCR-ABL1T315I leukemia cells isolated from BM and spleen of tumor-bearing mice, as assessed by intracellular flow cytometric staining for phospho-STAT5 and immunoblotting of tissue extracts for phospho-BCR-ABL1 and phospho-STAT5. Treatment of mice bearing Ba/F3-BCR-ABL1T315I leukemia cells with Rebastinib at 100 mg/kg once daily by oral gavage significantly prolonged their survival, while imatinib at 100 mg/kg twice daily is ineffective. In this aggressive allograft model, Rebastinib (DCC-2036) is as effective for treatment of BCR-ABLT315I leukemia as imatinib at 100 mg/kg twice daily is for treatment of BCR-ABL1native leukemia, and reduced the leukemia cell burden in the spleens of treated mice[1]. |
| Kinase Assay | Peripheral blood blasts from a patient with relapsed and refractory Ph+ B-ALL and detectable T315I mutation (allele frequency 40%) are incubated overnight (initial cell viability >90%) in IMDM supplemented with 100 μM 2-mecaptoethanol and 0.5% BSA, and either without drug or with Imatinib (1 μM) or Rebastinib (DCC-2036) (50, 200, and 1000 nM). After incubation, cells are lysed and protein extracts subjected to immunoblot analysis as described above. Peripheral blood mononuclear cells (7.5×105) from a patient with chronic phase CML and L298V mutation are incubated in varying concentrations of Rebastinib (DCC-2036) or DMSO for 3h, followed by lysis and immunoblot analysis[1]. |
| Cell Assay | Ba/F3 cells (3×103 cells/well) or primary Ph+ leukemia cells (5×104 cells/well) are plated in triplicate in 96-well plates containing test compounds (e.g., Rebastinib (DCC-2036)). After 72h, viable cells are quantified by resazurin or MTT assay. Results represent an average of at least three independent experiments[1]. |
| Animal Admin | Mice[1] Ba/F3 cells (1×106) transformed to interleukin-3 independence by transduction with either BCR-ABL1native or BCR-ABL1T315I retrovirus are injected intravenously into syngeneic Balb/c recipients. Beginning day 3 post-injection, mice are treated with Imatinib (100 mg/kg in water twice daily via oral gavage) or with Rebastinib (DCC-2036) (100 mg/kg in 0.5% CMC/1% Tween-80, once daily via oral gavage) or with vehicle (0.5% CMC/1% Tween-80) alone. For induction of CML-like leukemia, bone marrow (BM) from male Balb/c donor mice is harvested 4d after intravenous administration of 150 mg/kg 5-fluorouracil (5-FU), transduced with BCR-ABL1T315I retrovirus, and 5×105 cells injected intravenously into sublethally irradiated (400 cGy) Balb/c recipients. Beginning at d5 post-transplant, cohorts are treated once daily by oral gavage with vehicle alone, or Rebastinib (DCC-2036) at 100 mg/kg. For induction of B-cell acute lymphoblastic leukemia, BM from donors not pretreated with 5-FU is transduced once with BCR-ABL1T315I retrovirus and 1×106 cells injected into sublethally irradiated Balb/c recipients. Beginning at d8 post-transplant, cohorts are treated twice daily by oral gavage with vehicle alone, with Rebastinib (DCC-2036) at 60 mg/kg, with Imatinib at 100 mg/kg (in water), or with Dasatinib at 10 mg/kg (in 80 mM citric acid pH 3.1). |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 666.8±55.0 °C at 760 mmHg |
| Molecular Formula | C30H28FN7O3 |
| Molecular Weight | 553.587 |
| Flash Point | 357.0±31.5 °C |
| Exact Mass | 553.223755 |
| PSA | 126.55000 |
| LogP | 4.90 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.655 |
| Storage condition | -20℃ |
|
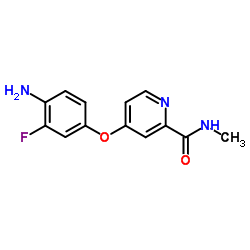
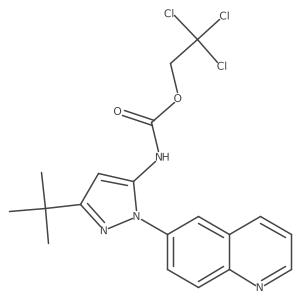
~45% 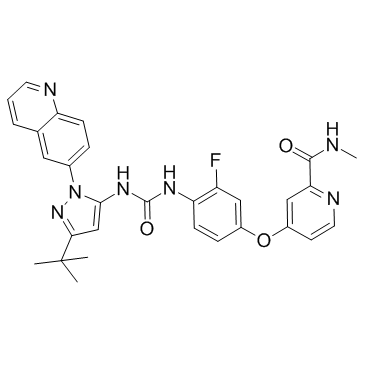
1020172-07-9 |
| Literature: DECIPHERA PHARMACEUTICALS, LLC; FLYNN, Daniel L.; PETILLO, Peter A.; KAUFMAN, Michael D. Patent: WO2013/36232 A2, 2013 ; Location in patent: Paragraph 00358 ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
|
~% 
1020172-07-9 |
| Literature: WO2013/36232 A2, ; |
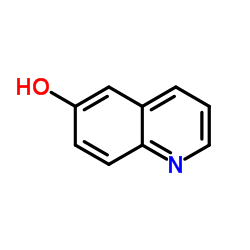
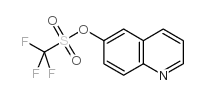
| Precursor 7 | |
|---|---|
| DownStream 0 | |