36545-53-6
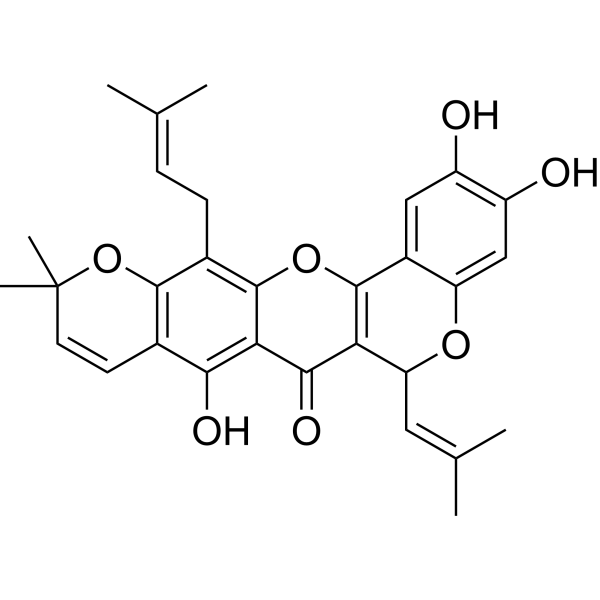
| Name | Cycloheterophyllin |
|---|---|
| Synonyms |
Acylated oxime isatin derivative,18
2,3,8-Trihydroxy-11,11-dimethyl-13-(3-methyl-2-buten-1-yl)-6-(2-methyl-1-propen-1-yl)-6H,7H,11H-chromeno[4,3-b]pyrano[3,2-g]chromen-7-one HMS2269P06 2,3,8-Trihydroxy-11,11-dimethyl-13-(3-methylbut-2-en-1-yl)-6-(2-methylprop-1-en-1-yl)-6H,7H,11H-chromeno[4,3-b]pyrano[3,2-g]chromen-7-one cycloheterphyllin |
| Description | Cycloheterophyllin is a flavonoid, that can be isolated from Artocarpus communis and A. heterophyllus. Cycloheterophyllin shows antiinflammatory activity[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 741.4±60.0 °C at 760 mmHg |
| Molecular Formula | C30H30O7 |
| Molecular Weight | 502.56 |
| Flash Point | 246.3±26.4 °C |
| Exact Mass | 502.199158 |
| PSA | 109.36000 |
| LogP | 8.12 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.680 |
| Hazard Codes | Xi |
|---|