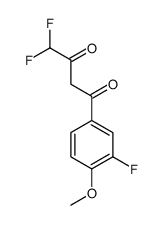
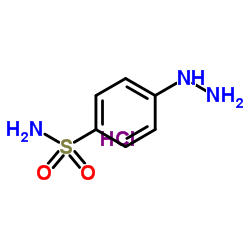
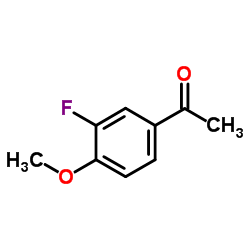
169590-41-4
| Name | deracoxib |
|---|---|
| Synonyms |
deracoxib
4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)pyrazol-1-yl]benzenesulfonamide 4-(3-(Difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1H-pyrazol-1-yl)benzenesulfonamide 4-[3-(Difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1H-pyrazol-1-yl]benzenesulfonamide |
| Description | Deracoxib, a selective cyclooxygenase-2 inhibitor, is a non-narcotic, non-steroidal anti-inflammatory drug (NSAID). IC50 Value: 70 to 150 uM(inhibition of 3 osteosarcoma cell lines) [1]Target: COXin vitro: Concentration of deracoxib required for 50% inhibition of cell viability (IC50) was reached in all 3 osteosarcoma cell lines and ranged from 70 to 150 microM, whereas the IC50 for piroxicam was only reached in the POS cell line at 500 microM. Neither deracoxib nor piroxicam induced sufficient toxicity in fibroblasts to reach an IC50. Exposure of osteosarcoma cells to cytotoxic concentrations of deracoxib and piroxicam did not result in DNA fragmentation [1]. Concomitant treatment of cells with piroxicam and deracoxib resulted in significant induction of apoptosis at lower concentrations and accumulation of cells in the G /G phase. Significant cytotoxic effects exhibited by the combination of piroxicam and deracoxib against canine mammary carcinoma cells in vitro suggest an attractive approach for the treatment of canine mammary carcinoma [2].in vivo: Perioperative administration of deracoxib to dogs at 1-2 mg/kg/day for 3 days significantly improves analgesia in the postoperative surgical period after soft tissue surgery [3]. Dogs were treated PO with deracoxib at a dosage of 3 mg/kg/d (1.36 mg/lb/d) as a single-agent treatment for TCC. Tumor response was assessed via radiography, abdominal ultrasonography, and ultrasonographic mapping of urinary bladder masses. Toxic effects of deracoxib administration in dogs were assessed through clinical observations and hematologic and biochemical analyses. 24 dogs for which tumor response was assessed, 4 (17%) had partial remission, 17 (71%) had stable disease, and 3 (13%) had progressive disease; initial response could not be assessed in 2 of 26 dogs. The median survival time was 323 days. Median time to progressive disease was 133 days. Renal, hepatic, and gastrointestinal abnormalities attributed to deracoxib administration were noted in 4% (1/26), 4% (1/26), and 19% (5/26) of dogs, respectively [4]. |
|---|---|
| Related Catalog | |
| Target |
COX-2 |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 575.5±60.0 °C at 760 mmHg |
| Molecular Formula | C17H14F3N3O3S |
| Molecular Weight | 397.372 |
| Flash Point | 301.9±32.9 °C |
| Exact Mass | 397.070801 |
| PSA | 95.59000 |
| LogP | 2.54 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.610 |
| Storage condition | -20℃ |
| HS Code | 2935009090 |
|---|
|
~89% 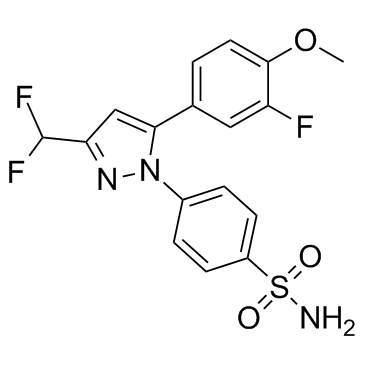
169590-41-4 |
| Literature: G.D. Searle and Co. Patent: US5760068 A1, 1998 ; |
|
~89% 
169590-41-4 |
| Literature: G. D. Searle and Co. Patent: US6342510 B1, 2002 ; Location in patent: Page column 28 - 29 ; |
|
~% 
169590-41-4 |
| Literature: US5892053 A1, ; |
|
~% 
169590-41-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 40, # 9 p. 1347 - 1365 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |