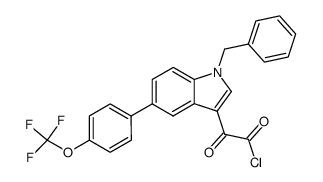
393105-53-8
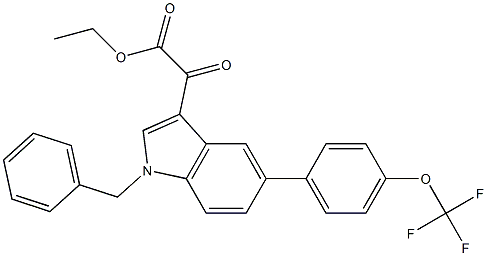
| Name | 2-[1-benzyl-5-[4-(trifluoromethoxy)phenyl]indol-3-yl]-2-oxoacetic acid |
|---|---|
| Synonyms |
2-{1-benzyl-5-[4-(trifluoromethoxy)phenyl]-1H-indol-3-yl}-2-oxoacetic acid
Tiplasinin PAI-039 UNII-L396QIB983 {1-Benzyl-5-[4-(trifluoromethoxy)phenyl]-1H-indol-3-yl}(oxo)acetic acid Tiplaxtinin Tiplasinin [USAN] 2-[1-benzyl-5-[4-(trifluoromethoxy)phenyl]indol-3-yl]-2-oxo-acetic aci d |
| Description | Tiplaxtinin is a selective and orally efficacious inhibitor of plasminogen activator inhibitor-1 (PAI-1) with IC50 of 2.7 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.7 μM (PAI-1)[1] |
| In Vitro | Tiplaxtinin (PAI-039), a small-molecule inhibitor of PAI-1 activity, on the urothelial cell lines. A significant inhibition in cellular proliferation is noted in T24 cells treated with Tiplaxtinin with the documentation of a favorable IC50 value of 43.7±6.3 μM and in UM-UC-14 cells 52.8±1.6 μM whereas the benign cell line, UROtsa, is noted to have a higher IC50 value of 70.3±0.1 μM. Notably, IC50 values of Tiplaxtinin in detached cells, 19.7±3.8 μM in T24, 44.5±6.5 μM in UM-UC-14, and 31.6±6.1 μM in UROtsa, are significantly lower than the IC50 values calculated for cells cultured in the presence of Tiplaxtinin under attached conditions[2]. |
| In Vivo | In the vena cava protocol, Tiplaxtinin (PAI-039) pretreatment significantly reduces thrombus weight at Tiplaxtinin doses of 3, 10 and 30 mg/kg. When Tiplaxtinin is dosed in a treatment paradigm 4 h after stable arterial and venous thrombosis, a significant reduction in thrombus weight is observed 24 h later at Tiplaxtinin doses of 3, 10 and 30 mg/kg[1]. Tiplaxtinin (PAI-039) is administered by oral gavage to athymic mice bearing human bladder cancer cell line T24 xenografts and human cervical cancer HeLa cell xenografts. The subcutaneous tumor growth of both T24 and HeLa cell xenografts treated with Tiplaxtinin is markedly reduced compared with untreated controls. Specifically, at the end of the study, control T24 xenografts are noted to be 1,150±302 mm3 compared with 593±328 mm3 for T24 xenograft tumors treated with 5 mg/kg Tiplaxtinin (P<0.0001) and 627±248 mm3 for T24 xenografts treated with 20 mg/kg (P<0.0001)[2]. Tiplaxtinin (1, 3, and 10 mg/kg) is subjected to electrolytic injury of the coronary artery. Tiplaxtinin (PAI-039) causes prolongation in time to coronary occlusion (control, 31.7±6.3 min; 3 mg/kg Tiplaxtinin, 66.0±6.4 min; 10 mg/kg, 56.7±7.4 min; n=5-6; p<0.05) and a reduced thrombus weight (control, 7.6±1.5 mg; 10 mg/kg Tiplaxtinin, 3.6±1.0 mg; p<0.05)[3]. |
| Cell Assay | T24, UM-UC-14, UROtsa, and HeLa cells are plated in 96-well dishes in triplicate at 1×103 cells per well and allowed to adhere for 24 hours. Subsequently, Tiplaxtinin is added to the wells and allowed to incubate at the indicated concentrations. Cellular proliferation is determined by CellTiter-Glo Luminescent Cell Viability Assay at 24 hours, and IC50 of Tiplaxtinin is determined in Graphpad Prism. Luminescence is measured using a FLUOstar OPTIMA Reader[2]. |
| Animal Admin | Rats[1] Male Sprague-Dawley rats (250-350 g) are dosed orally with either vehicle or Tiplaxtinin (0.3-3 mg/kg) on the morning of the experiment, then anesthetized with sodium-pentobarbital (50 mg/kg, i.p.). A midline incision is made along the neck, and the right and left carotid arteries and jugular veins are exposed. The left jugular vein is catheterized for tPA delivery and blood sampling. The right carotid artery is cannulated with PE-60 tubing filled with 3.8% sodium citrate solution, and interfaced to a saline-filled pressure transducer for monitoring of heart rate and blood pressure. The left carotid artery is used for vascular injury induction and thrombosis. An ultrasonic flow probe is placed on the left carotid artery, and baseline blood flow is recorded. A 3-mm section of PE-60 tubing is sectioned longitudinally, and a piece of filter paper saturated with 25% FeCl3 is inserted into the tubing. Flow is monitored in the damaged vessel during tPA infusion and for an additional 20 min afterwards. At the end of the experiment, the arterial thrombus is excised and weighed. Mice[2] Mice bearing bladder xenografts and mice bearing cervical xenografts are divided randomly into three groups (control, 5 mg/kg of Tiplaxtinin, and 20 mg/kg of Tiplaxtinin) and treatment is initiated. Each group is composed of at least 10 mice. No toxicity or weight loss is noted in any of the treatment groups. Tiplaxtinin (100 μL diluted in corn oil) is administered via oral gavage daily (Monday-Friday) for 5 weeks. Control mice received vehicle alone on the same schedule. Tumor volumes are measured weekly with digital calipers and calculated. After 5 weeks, the mice are sacrificed, tumors resected, and analyzed by immunohistochemical staining. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 588.0±50.0 °C at 760 mmHg |
| Molecular Formula | C24H16F3NO4 |
| Molecular Weight | 439.383 |
| Flash Point | 309.4±30.1 °C |
| Exact Mass | 439.103149 |
| PSA | 68.53000 |
| LogP | 5.32 |
| Appearance | light yellow solid |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.593 |
| Storage condition | -20℃ |
| Hazard Statements | H413 |
|---|---|
| RIDADR | NONH for all modes of transport |
| Precursor 5 | |
|---|---|
| DownStream 0 | |