71030-37-0
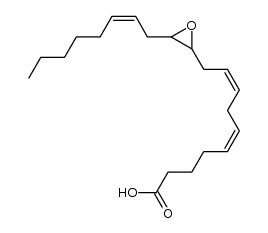
| Name | (5Z,8Z,10E,14Z)-12-hydroxyicosa-5,8,10,14-tetraenoic acid |
|---|---|
| Synonyms |
(±)12-HETE
12-Hydroxyeicosatetraenoic acid (5Z,8Z,10E,14Z)-12-hydroxyicosa-5,8,10,14-tetraenoic acid (5Z,8Z,10E,14Z)-12-hydroxyicosatetraenoic acid (5Z,8Z,10E,14Z)-12-Hydroxy-5,8,10,14-icosatetraenoic acid |
| Description | 12-HETE, a major metabolic product of arachidonic acid using 12-LOX catalysis, inhibits cell apoptosis in a dose-dependent manner. 12-HETE promotes the activation and nuclear translocation of NF-κB through the integrin-linked kinase (ILK) pathway[1].12-HETE has both anti-thrombotic and pro-thrombotic effects[2]. 12-HETE is a neuromodulator[3]. |
|---|---|
| Related Catalog | |
| In Vitro | 12-HETE participates in the inhibition of cell apoptosis by activating the ILK/NF-κB pathway, implying an important underlying mechanism that promotes the survival of ovarian cancer cells. 12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-κB pathway in ovarian cancer. 12-HETE protects against cell apoptosis in ovarian cancer cells in a concentration-dependent manner. 12-HETE (1 µM) significantly decreases the activation of caspase-3 induced by serum deprivation (SD).12-HETE represses the increased activity of caspase-3 induced by SD in a concentration-dependent manner, with an IC50 value of 1.13 µM[1]. 12-HETE (1 µM) facilitates the activation and nuclear translocation of NF-κB via ILK in ovarian cancer cells[1]. 12-HETE inhibits insulin secretion, reduces metabolic activity and induces cell death in human islets. 12-HETE increases bovine platelet aggregation induced by thrombin and inhibits prostaglandin E1-induced elevation of intracellular cAMP levels. 12-HETE inhibits washed platelet (WP) aggregation[2]. The neuronal effects of 12-HETE include attenuation of calcium influx and glutamate release as well as inhibition of AMPA receptor (AMPA-R) activation[3]. Cell Viability Assay[1] Cell Line: Ovarian cancer OVCAR-3 and SKOV3 cells Concentration: 0, 0.2, 0.5, and 1 µM Incubation Time: 0, 24, 48, 72, and 96 hours Result: Inhibited the decrease in cell viability induced by SD in a dose-dependent manner. 1 µM 12-HETE treatment significantly mitigated the decrease in cell viability under conditions of SD. Western Blot Analysis[1] Cell Line: Ovarian cancer OVCAR-3 and SKOV3 cells Concentration: 1 µM Incubation Time: Result: Led to increased levels of NF-κB p65 phosphorylation. Caused a significant increase in the protein levels of nuclear NF-κB p65, which was accompanied by decreased levels of NF-κB p65 in the cytoplasm. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 487.7±45.0 °C at 760 mmHg |
| Molecular Formula | C20H32O3 |
| Molecular Weight | 320.466 |
| Flash Point | 262.8±25.2 °C |
| Exact Mass | 320.235138 |
| PSA | 17.07000 |
| LogP | 5.45 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.514 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| HS Code | 2918199090 |
|---|
| Precursor 8 | |
|---|---|
| DownStream 0 | |
| HS Code | 2918199090 |
|---|---|
| Summary | 2918199090 other carboxylic acids with alcohol function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |