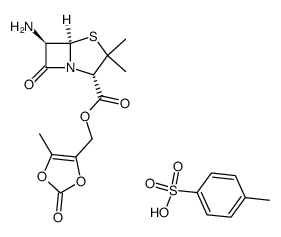
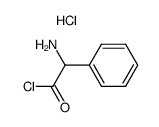
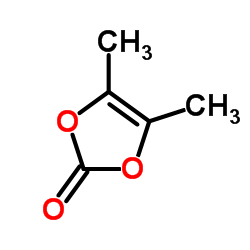
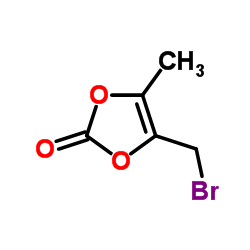
80734-02-7
| Name | (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl (2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate,hydrochloride |
|---|---|
| Synonyms |
lenampicillin hydrochloride
Lenampicillin hydrochloride (JP16) Valacillin (TN) Lenampicillin (hydrochloride) |
| Description | Lenampicillin (hydrochloride) is the efficient prodrug of ampicillin (ABPC ) in terms of the enhancement of absorption and decrease of side effects.In vivo : The intestinal absorption of LAPC is satisfactory in view of the urinary excretion of metabolites, accounting for 93% of dose in human, 74% in dogs and 55% in rats, respectively. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 717.4ºC at 760 mmHg |
|---|---|
| Molecular Formula | C21H24ClN3O7S |
| Molecular Weight | 497.95 |
| Flash Point | 387.7ºC |
| PSA | 170.38000 |
| LogP | 1.86060 |
| Storage condition | 2-8℃ |
|
~75% 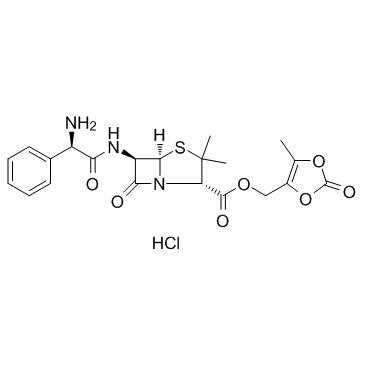
80734-02-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 32, # 11 p. 4316 - 4322 |
|
~% 
80734-02-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 32, # 6 p. 2241 - 2248 |
|
~% 
80734-02-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 32, # 6 p. 2241 - 2248 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |