51116-00-8
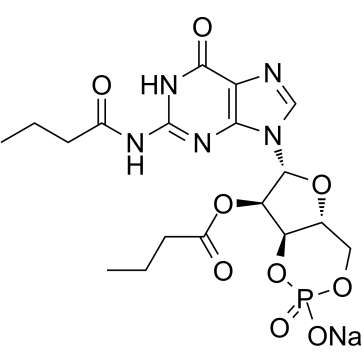
| Name | N2,2′-O-Dibutyrylguanosine 3′,5′-cyclic monophosphate sodium salt hydrate |
|---|---|
| Synonyms |
sodium,[6-[2-(butanoylamino)-6-oxo-3H-purin-9-yl]-2-oxido-2-oxo-4a,6,7,7a-tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-7-yl] butanoate
Butanoic acid, (4aR,6R,7R,7aR)-6-[1,6-dihydro-6-oxo-2-[(1-oxobutyl)amino]-9H-purin-9-yl]tetrahydro-2-hydroxy-2-oxido-4H-furo[3,2-d]-1,3,2-dioxaphosphorin-7-yl ester, sodium salt (1:1) Sodium (4aR,6R,7R,7aR)-6-[2-(butyrylamino)-6-oxo-1,6-dihydro-9H-purin-9-yl]-7-(butyryloxy)tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-2-olate 2-oxide |
| Description | Dibutyryl-cGMP sodium (Bt2cGMP sodium) is a cell-permeable cGMP analogue. Dibutyryl-cGMP sodium preferentially activates cGMP-dependent protein kinase (PKG). Dibutyryl-cGMP sodium inhibits the release of [3H]-arachidonic acid from γ thrombin-stimulated human platelets. Dibutyryl-cGMP sodium induces peripheral antinociception via activation of ATP-sensitive K+ channels[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
cGMP-dependent protein kinase (PKG)[1]; ATP-sensitive K+ channels[3] |
| In Vitro | Dibutyryl-cGMP is able to induce process elongation and branching in astrocytes resulting from a rapid, reversible and concentration-dependent redistribution of glial fibrillary acidic protein (GFAP) and actin filaments without significant change in protein levels[1]. When cells are co-incubated with Dibutyryl-cGMP (100 μM) stress fibre formation is prevented and cells acquired a stellate morphology in cerebellar astrocytes[1]. In cells treated with Dibutyryl-cGMP (100 μM, 2 h) the particulate fraction is nearly devoid of RhoA protein. Dibutyryl-cGMP prevents RhoA-membrane association[1]. Using the scratchwound model, the size of the wound is significantly smaller in cells treated with Dibutyryl-cGMP after the wound indicating that dbcGMP accelerates wound closure[1]. |
| In Vivo | Dibutyryl-cGMP (50-200 μg/paw; subcutaneous injection; male Wistar rats) treatment antagonizes the hyperalgesic effect of PGE2 in a dose-dependent manner. Maximal antinociceptive effect of DbcGMP is at 1 h after administration and last for plus 2 h[3]. Animal Model: Male Wistar rats (180- 250 g) injection with Prostaglandin E2 (PGE2)[3] Dosage: 50 μg/paw, 75 μg/paw, 100 μg/paw and 200 μg/paw Administration: Subcutaneous injection Result: Antagonized the hyperalgesic effect of PGE2 (2 μg/paw), in a dose-dependent manner. |
| References |
| Molecular Formula | C18H23N5NaO9P |
|---|---|
| Molecular Weight | 507.367 |
| Exact Mass | 507.113098 |
| PSA | 196.60000 |
| LogP | 1.49450 |