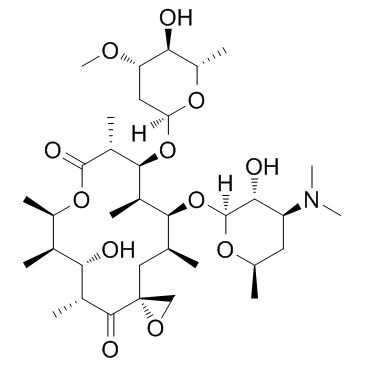
2751-09-9
| Name | troleandomycin |
|---|---|
| Synonyms |
tao
TRIACETYLOLEANDOMYCIN TROLEANDOMYCIN Taocin evramicina cyclamycin Tri-O-acetyl-oleandomycin EINECS 220-392-9 Mathromycin T OLEANDOMYCIN TRIACETATE |
| Description | Troleandomycin (Triacetyloleandomycin), a macrolide acrolide antibiotic, is a selective CYP3A inhibitor. Troleandomycin is an oral corticosteroid for asthma study[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Troleandomycin markedly inhibits 6β-hydroxylation of testosterone, 25- and 26-hydroxylations of 5β-cholestane-3α,7α,12α-triol and 23R-, 24R-, 24S-, and 27-hydroxylations of 5β-cholestane-3α,7α,12α,25-tetrol in both recombinant CYP3A4 and microsomes, but IC50 values for microsomes are somewhat higher than those for recombinant CYP3A4[1]. |
| In Vivo | Troleandomycin markedly suppresses these microsomal side chain hydroxylations in both mouse and human livers in a dose-dependent manner[2]. Animal Model: SD female rats[1]. Dosage: 500 mg/kg. Administration: A single oral dose. Result: Markedly elevated the Cmax and AUC0-6 of simvastatin by 9.5- and 10.2-fold, respectively. |
| References |
| Density | 1.19g/cm3 |
|---|---|
| Boiling Point | 812.5ºC at 760mmHg |
| Melting Point | 170 °C (lit.) |
| Molecular Formula | C41H67NO15 |
| Molecular Weight | 813.96800 |
| Flash Point | 445.2ºC |
| Exact Mass | 813.45100 |
| PSA | 184.19000 |
| LogP | 3.62090 |
| Vapour Pressure | 1.82E-26mmHg at 25°C |
| Index of Refraction | 1.515 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | 36/37/38 |
| Safety Phrases | S22-S24/25 |
| WGK Germany | 2 |
| RTECS | RJ9900000 |
|
~% 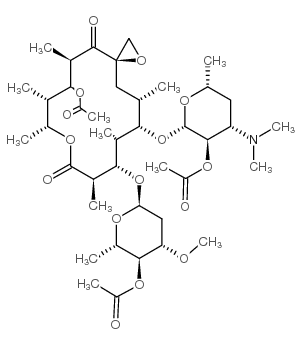
2751-09-9 |
| Literature: GB877730 , ; Antibiotics Annual, p. 476,479 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |