7182-54-9
| Name | Monactin |
|---|---|
| Synonyms |
Nonactin,5-demethyl-5-ethyl
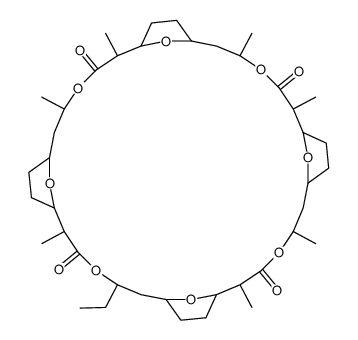
5-ethyl-2,11,14,20,23,29,32-heptamethyl-4,13,22,31,37,38,39,40-octaoxapentacyclo[32.2.1.17,10.116,19.125,28]tetracontane-3,12,21,30-tetrone Akd-1B |
| Description | Monactin is a mactrotetralide antibiotic and a non-selective ionophore for monovalent cations, including potassium, sodium, and lithium. Monactin is isolated from Streptomyces and has antiproliferative activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Monactin displays strong antiproliferative activity against A2780 ovarian cancer cells (IC50 0.13 μM), A2058 melanoma cells (0.02 μM), and H522-T1 non small-cell cancer lung cells (0.01 μM)[3]. Monactin inhibits TCF/β-catenin transcriptional activity[4]. At a concentration of 10 μM, nigericin and monactin inhibited growth of Streptococcus faecalis, and the inhibition was reversed by addition of excess K(+)[5]. |
| In Vivo | Monactin is potent uncoupler of oxidative phosphorylation and inducer of ATP hydrolysis in rat liver mitochondria[1]. |
| References |
| Density | 1.035g/cm3 |
|---|---|
| Boiling Point | 896.9ºC at 760 mmHg |
| Molecular Formula | C41H66O12 |
| Molecular Weight | 750.95600 |
| Flash Point | 353.8ºC |
| Exact Mass | 750.45500 |
| PSA | 142.12000 |
| LogP | 6.41210 |
| Index of Refraction | 1.451 |