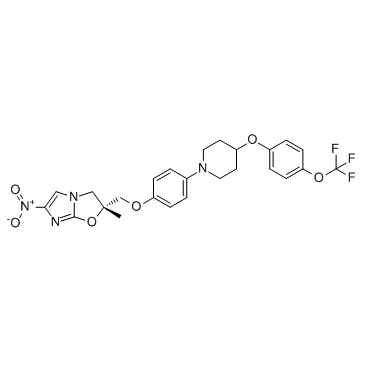
681492-22-8
| Name | (2R)-2-methyl-6-nitro-2-[[4-[4-[4-(trifluoromethoxy)phenoxy]piperidin-1-yl]phenoxy]methyl]-3H-imidazo[2,1-b][1,3]oxazole |
|---|---|
| Synonyms |
Delamanid
(2R)-2-methyl-6-nitro-2-[(4-{4-[4-(trifluoromethoxy)phenoxy]piperidin-1-yl}phenoxy)methyl]-2,3-dihydroimidazo[2,1-b][1,3]oxazole (2R)-2-Methyl-6-nitro-2-[(4-{4-[4-(trifluoromethoxy)phenoxy]-1-piperidinyl}phenoxy)methyl]-2,3-dihydroimidazo[2,1-b][1,3]oxazole UNII-8OOT6M1PC7 OPC-67683 |
| Description | Delamanid, a newer mycobacterial cell wall synthesis inhibitor, inhibits the synthesisi of mucolic acids, cruciala component of the cell wall of the Mycobacterium tuberculosis complex.In vitro: inhibits the synthesisi of mucolic acids, cruciala component of the cell wall of the Mycobacterium tuberculosis complex.[1]In in-vitro studies, delamanid shows more potent antibacterial activity against drug-susceptible and drug-resistant strains of M. tuberculosis.[2] Delamanid do not affect rifampin, pyrazinamide, and isoniazid exposure; the ethambutol AUCτ and Cmax values are about 25% higher with delamanid coadministration. [3] In vivo: Twice-daily oral dosing of delamanid at 30 mg kg-1 for 5 days resulted in sterile cures in a mouse model of VL. [4] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 653.7±65.0 °C at 760 mmHg |
| Molecular Formula | C25H25F3N4O6 |
| Molecular Weight | 534.484 |
| Flash Point | 349.1±34.3 °C |
| Exact Mass | 534.172607 |
| PSA | 103.80000 |
| LogP | 4.75 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.611 |
| Storage condition | -20℃ |