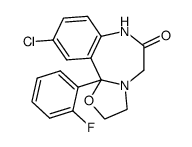
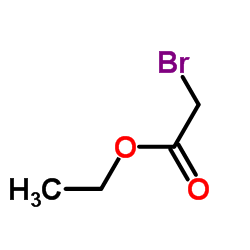
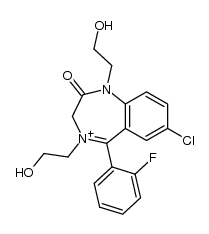
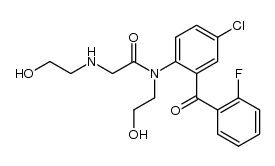
27060-91-9
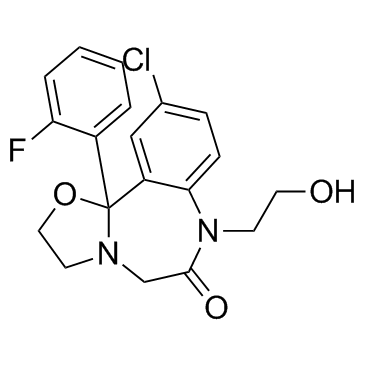
| Name | 10-chloro-11b-(2-fluorophenyl)-7-(2-hydroxyethyl)-3,5-dihydro-2H-[1,3]oxazolo[3,2-d][1,4]benzodiazepin-6-one |
|---|---|
| Synonyms |
10-Chloro-11b-(o-fluorophenyl)-2,3,7,11b-tetrahydro-7-(2-hydroxyethyl)oxazolo(3,2-d)(1,4)benzodiazepin-6(5H)-one
Oxazolo(3,2-d)(1,4)benzodiazepin-6(5H)-one,10-chloro-11b-(o-fluorophenyl)-2,3,7,11b-tetrahydro-7-(2-hydroxyethyl) 10-Chloro-11b-(2-fluorophenyl)-7-(2-hydroxyethyl)-2,3,5,11b-tetrahydrooxazolo[3,2-d][1,4]benzodiazepin-6(7H)-one MS-4101 flutazolam Flutazolam [INN:JAN] Flutazolamum [INN-Latin] 10-Chloro-11b-(2-fluorophenyl)-2,3,7,11b-tetrahydro-7-(2-hydroxyethyl)oxazolo(3,2-d)(1,4)benzodiazepin-6(5H)-one Flutazolamum |
| Description | Flutazolam (MS 4101; Ro 7-6102) is a medicine acts on benzodiazepine receptors of the brain and relieves anxiety or tension. |
|---|---|
| Related Catalog | |
| Target |
Benzodiazepine receptor[1] |
| In Vitro | MS-4101 suppresses hyperemotionality in septal rats, fighting behavior in long-term isolated mice and pentylenetetrazol convulsion and potentiated thiopental sleep. These effects of MS-4101 are the same in potency as those of diazepam. MS-4101 inhibits considerably both scratching and head-twitch induced by mescaline in mice[1]. |
| References |
| Density | 1.47g/cm3 |
|---|---|
| Boiling Point | 601.9ºC at 760mmHg |
| Molecular Formula | C19H18ClFN2O3 |
| Molecular Weight | 376.80900 |
| Flash Point | 317.8ºC |
| Exact Mass | 376.09900 |
| PSA | 53.01000 |
| LogP | 2.35420 |
| Vapour Pressure | 2.44E-15mmHg at 25°C |
| Index of Refraction | 1.672 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
27060-91-9 |
| Literature: Hoffmann-La Roche Inc. Patent: US3965151 A1, 1976 ; |
|
~% 
27060-91-9 |
| Literature: Hoffmann-La Roche Inc. Patent: US3965151 A1, 1976 ; |
|
~% 
27060-91-9 |
| Literature: Kuruno; Kamiya; Kuwayama; Jinno; Yashiro; Ikeda Chemical and Pharmaceutical Bulletin, 1987 , vol. 35, # 9 p. 3831 - 3837 |
|
~% 
27060-91-9 |
| Literature: Kuwayama; Kurono; Muramatsu; Yashiro; Ikeda Chemical and Pharmaceutical Bulletin, 1986 , vol. 34, # 1 p. 320 - 326 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |