246852-12-0
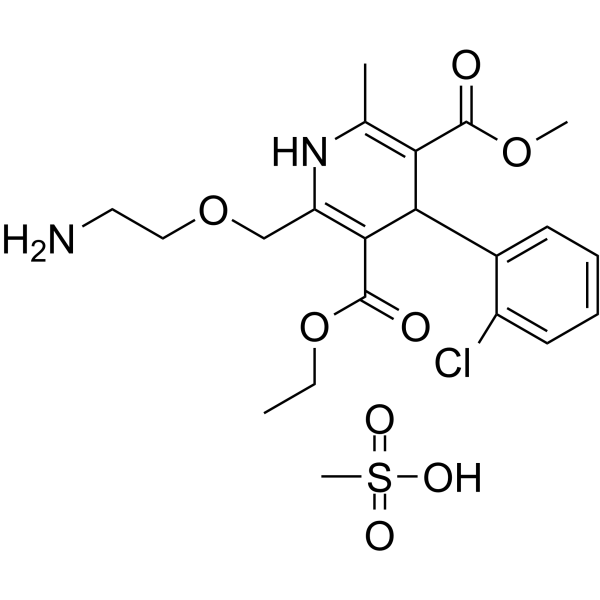
| Name | 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate,methanesulfonic acid |
|---|---|
| Synonyms |
3,5-Pyridinedicarboxylic acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl ester, methanesulfonate (1:1)
3-Ethyl 5-methyl 2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate methanesulfonate 3-Ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydro-3,5-pyridinedicarboxylate methanesulfonate (1:1) Norvasc UNII-291Y33EZHA Amlodipine Mesilate Amlodipine Mesylate UNII:291Y33EZHA 3-Ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate methanesulfonate (1:1) |
| Description | Amlodipine mesylate, an antianginal agent and an orally active dihydropyridine calcium channel blocker, works by blocking the voltage-dependent L-type calcium channels, thereby inhibiting the initial influx of calcium. Amlodipine mesylate can be used for the research of high blood pressure and cancer[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
L-type calcium channel |
| In Vitro | Amlodipine mesylate (20-40 μM; 48 h) reduces BrdU incorporation to 68.6% and 26.3% at concentrations of 20 and 30 μM in A431 cells, respectively[3]. Amlodipine mesylate (30 μM; pretreated for 1 h) significantly attenuates the uridine 5′-triphosphate (UTP)-induced increases of [Ca2+]i in A431 cells[3]. Amlodipine mesylate (30 μM) inhibits the store-operated Ca2+influx evoked by Thapsigargin in Fluo-3-loaded cells[3]. |
| In Vivo | Amlodipine mesylate (5 mg/kg/day; s.c. for 2 weeks) significantly decreases systolic blood pressure (SBP) in VSMC ATP2B1 KO mice[4]. Amlodipine mesylate (10 mg/kg; i.p. once daily for 20 days) causes a significant retardation of tumor growth and prolongs the survival of A431 tumor-bearing mice[3]. Animal Model: ATP2B1loxP/loxP mice[4] Dosage: 5 mg/kg/day Administration: Subcutaneously implanted osmotic pump for 2 weeks Result: Significantly decreased the blood pressure. |
| References |
| Molecular Formula | C21H29ClN2O8S |
|---|---|
| Molecular Weight | 504.982 |
| Exact Mass | 504.133301 |
| PSA | 162.63000 |
| LogP | 3.88020 |
| Hazard Codes | Xn |
|---|---|
| HS Code | 2933399090 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |