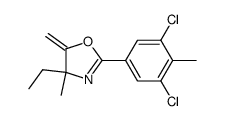
156052-68-5
| Name | zoxamide |
|---|---|
| Synonyms |
3,5-Dichloro-N-(1-chloro-3-methyl-2-oxopentan-3-yl)-4-methylbenzamide
N-[3'-(1'-chloro-3'-methyl-2'-oxopentan)]-3,5-dichloro-4-methylbenzamide rac-3,5-dichloro-N-[(3R)-1-chloro-3-methyl-2-oxopentan-3-yl]-4-methylbenzamide 3,5-Dichloro-N-(1-chloro-3-methyl-2-oxo-3-pentanyl)-4-methylbenzamide 3,5-dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxo-propyl)-4-methyl-benzamide zoxamide Zoxamid 3,5-Dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-p-toluamide 3,5-dichloro-N-(3-chloro-l-ethyl-l-methyl-2-oxopropyl)-4-methylbenzamide (S)-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-3.5-dichloro-4-methylbenzamide Zoxium rh-7281 3,5-Dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-4-methylbenzamide (RS)-3,5-Dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-p-toluamide N-(1-chloro-3-methyl-2-oxopent-3-yl)-3,5-dichloro-4-methylbenzamide |
| Description | Zoxamide (RH-7281) is an oomycete fungicide. Zoxamide arrests nuclear division in Phytophthora capsici germlings and destroyed the microtubule cytoskeleton[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 415.4±45.0 °C at 760 mmHg |
| Melting Point | 159.5-161ºC |
| Molecular Formula | C14H16Cl3NO2 |
| Molecular Weight | 336.64 |
| Flash Point | 205.0±28.7 °C |
| Exact Mass | 335.024658 |
| PSA | 46.17000 |
| LogP | 4.84 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.545 |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H319-H410 |
| Precautionary Statements | P273-P280-P305 + P351 + P338-P333 + P313-P391-P501 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| RIDADR | UN 3077 9 / PGIII |
|
~89% 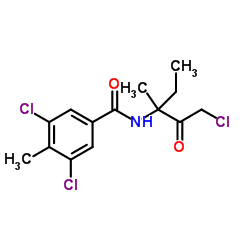
156052-68-5 |
| Literature: ROHM AND HAAS COMPANY Patent: EP952143 A1, 1999 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |