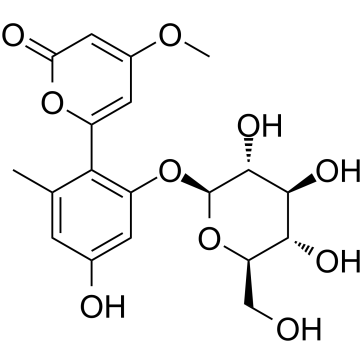
59163-53-0
| Name | 6-(2,4-dihydroxy-6-methylphenyl)-4-methoxypyran-2-one |
|---|---|
| Synonyms |
4-methoxy-6-(2',4'-dihydroxy-6'-methylphenyl)pyran-2-one
Aloenin-Aglycon 6-(2',4'-dihydroxy-6'-methylphenyl)-4-methoxy-2-pyrone 6-(2,4-dihydroxy-6-methyl-phenyl)-4-methoxy-pyran-2-one 2H-Pyran-2-one,6-(2,4-dihydroxy-6-methylphenyl)-4-methoxy aloenin aglycone |
| Description | Aloenin aglycone (compound 13) is an NF-κB inhibitor that can be isolated from aloe exudate. Aloenin aglycone inhibits TNFα-induced NF-κB transcriptional activity (IC50: 18.7 μM). Aloenin aglycone (10 μM) also reduced inducible nitric oxide synthase (iNOS) and intercellular adhesion molecule 1 (ICAM-1) gene expression after treatment of HepG2 cells with 10 ng/mL TNFα[1]. |
|---|---|
| Related Catalog | |
| Target |
intercellular adhesion molecule-1 (ICAM-1)[1] |
| References |
| Molecular Formula | C13H12O5 |
|---|---|
| Molecular Weight | 248.23100 |
| Exact Mass | 248.06800 |
| PSA | 79.90000 |
| LogP | 2.03500 |
|
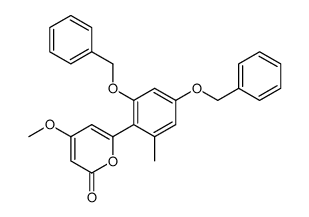
~95% 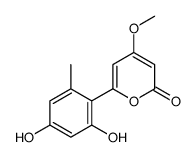
59163-53-0 |
| Literature: Bringmann; Schneider 1985 , vol. 1985, # 4 p. 765 - 774 |
|
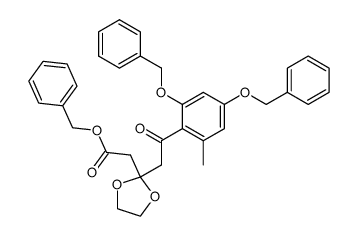
~% 
59163-53-0 |
| Literature: Bringmann; Schneider 1985 , vol. 1985, # 4 p. 765 - 774 |
|
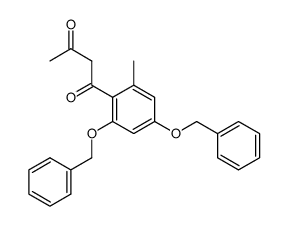
~% 
59163-53-0 |
| Literature: Bringmann; Schneider 1985 , vol. 1985, # 4 p. 765 - 774 |
|
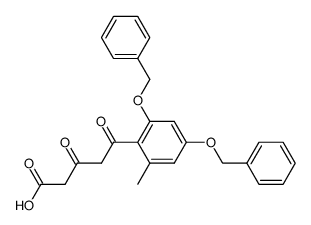
~% 
59163-53-0 |
| Literature: Bringmann; Schneider 1985 , vol. 1985, # 4 p. 765 - 774 |
|
~% 
59163-53-0 |
| Literature: Bringmann; Schneider 1985 , vol. 1985, # 4 p. 765 - 774 |
|
~% 
59163-53-0 |
| Literature: Bringmann; Schneider 1985 , vol. 1985, # 4 p. 765 - 774 |
|
~% 
59163-53-0 |
| Literature: Bringmann; Schneider 1985 , vol. 1985, # 4 p. 765 - 774 |
|
~% 
59163-53-0 |
| Literature: Conner, John M.; Gray, Alexander I.; Reynolds, Tom; Waterman, Peter G. Phytochemistry (Elsevier), 1987 , vol. 26, # 11 p. 2995 - 2998 |