2059148-82-0
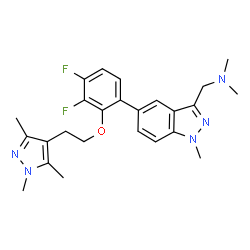
| Name | 1-(5-{3,4-Difluoro-2-[2-(1,3,5-trimethyl-1H-pyrazol-4-yl)ethoxy]phenyl}-1-methyl-1H-indazol-3-yl)-N,N-dimethylmethanamine |
|---|---|
| Synonyms | 1-(5-{3,4-Difluoro-2-[2-(1,3,5-trimethyl-1H-pyrazol-4-yl)ethoxy]phenyl}-1-methyl-1H-indazol-3-yl)-N,N-dimethylmethanamine |
| Description | IMP-1088 is a potent, selective human N-myristoyltransferase with IC50 of less than 1 nM for both HsNMT1 and HsNMT2, inhibits Rhinoviruses (RVs) capsid myristoylation in cells; pharmacological and rapidly inhibits host-cell N-myristoylation, potently and efficiently block RV replication (IC50=17 nM) without cytotoxicity; potently blocks a key step in viral capsid assembly, to deliver a low nanomolar antiviral activity against multiple RV strains, poliovirus and foot and-mouth disease virus, and protection of cells against virus-induced killing. |
|---|---|
| References | References 1. Aurélie Mousnier, et al. Fragment-derived inhibitors of human N-myristoyltransferase block capsid assembly and replication of the common cold virus. Nature Chemistry (2018). doi:10.1038/s41557-018-0039-2. View Related Products by Target Rhinovirus |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 566.9±50.0 °C at 760 mmHg |
| Molecular Formula | C25H29F2N5O |
| Molecular Weight | 453.527 |
| Flash Point | 296.6±30.1 °C |
| Exact Mass | 453.234009 |
| LogP | 3.73 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.595 |