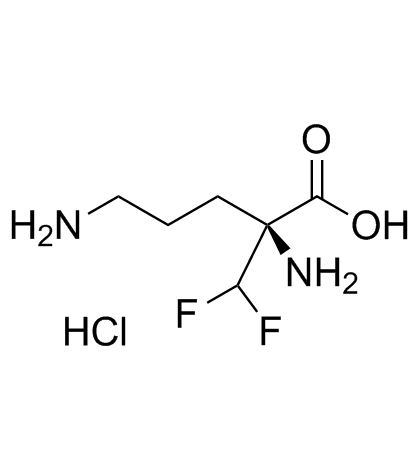
69955-42-6
| Name | L-Eflornithine monohydrochloride |
|---|
| Description | L-Eflornithine monohydrochloride (L-DFMO monohydrochloride) is an enantiomer of Eflornithine. L-Eflornithine is an irreversible ornithine decarboxylase (ODC) inhibitor with a KD of 1.3±0.3 µM, and a Kinact of 0.15±0.03 min-1[1]. |
|---|---|
| Related Catalog | |
| Target |
KD:1.3±0.3 µM (Ornithine decarboxylase, ODC)[1] |
| In Vitro | Eflornithine (D/L-DFMO) is an inhibitor of ODC, the first enzyme in eukaryotic polyamine biosynthesis. Both enantiomers of Eflornithine (DFMO) irreversibly inactivate ODC. Both Eflornithine enantiomers (L-Eflornithine and D-Eflornithine) suppress ODC activity in a time- and concentration-dependent manner. The inhibitor dissociation constant (KD) values for the formation of enzyme-inhibitor complexes are 28.3±3.4, 1.3±0.3 and 2.2±0.4 µM respectively for D-Eflornithine, L-Eflornithine and Eflornithine. The inhibitor inactivation constants (Kinact) for the irreversible step were 0.25±0.03, 0.15±0.03 and 0.15±0.03 min-1 respectively for D-Eflornithine, L-Eflornithine and Eflornithine. Treatment of human colon tumour-derived HCT116 cells with either L-Eflornithine or D- Eflornithine decreases the cellular polyamine contents in a concentration-dependent manner[1]. The enantiomers display different potencies in vitro, with the L-enantiomer having up to a 20-fold higher affinity for the target enzyme ornithine decarboxylase[2]. The L-Eflornithine also appears to be more potent in cultured T.brucei gambiense parasites[2]. |
| In Vivo | The more potent L-Eflornithine is present at much lower concentrations in both plasma and cerebrospinal fluid (CSF) than those of the D-Eflornithine. The plasma concentrations of L-Eflornithine are on average 52% of the D-enantiomer concentrations. The typical oral clearances of L-Eflornithine and D-eflornithine are 17.4 and 8.23 liters/h, respectively[2]. |
| References |
| Molecular Formula | C6H13ClF2N2O2 |
|---|---|
| Molecular Weight | 218.63 |