73215-92-6
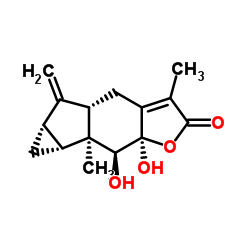
| Name | (4aS,5aS,6aR,6bS,7S,7aR)-7,7a-Dihydroxy-3,6b-dimethyl-5-methylene-4a,5,5a,6,6a,6b,7,7a-octahydrocyclopropa[2,3]indeno[5,6-b]furan-2(4H)-one |
|---|---|
| Synonyms | (4aS,5aS,6aR,6bS,7S,7aR)-7,7a-Dihydroxy-3,6b-dimethyl-5-methylene-4a,5,5a,6,6a,6b,7,7a-octahydrocyclopropa[2,3]indeno[5,6-b]furan-2(4H)-one |
| Description | Chloranthalactone E (compound 6), a labdane diterpene, can be isolated from the aerial parts of Chloranthus serratus. Chloranthalactone E inhibits NO production in LPS-activated RAW 264.7 macrophages[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 458.8±45.0 °C at 760 mmHg |
| Molecular Formula | C15H18O4 |
| Molecular Weight | 262.301 |
| Flash Point | 175.6±22.2 °C |
| Exact Mass | 262.120514 |
| LogP | 1.07 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.623 |
| Storage condition | 2-8℃ |