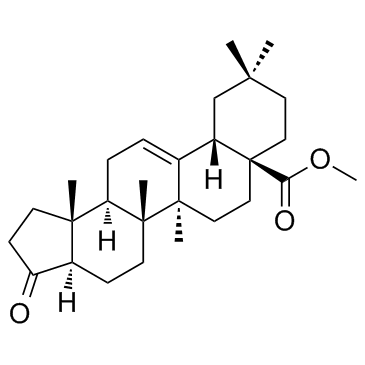
211516-63-1
| Name | Oleanolic acid derivative 2 |
|---|---|
| Synonyms |
7aH-Cyclopenta[a]chrysene-7a-carboxylic acid, 1,2,3,3a,4,5,5a,5b,6,7,8,9,10,11,11a,13,13a,13b-octadecahydro-5a,5b,10,10,13b-pentamethyl-3-oxo-, methyl ester, (3aR,5aR,5bS,7aS,11aS,13aR,13bR)-
Methyl (3aR,5aR,5bS,7aS,11aS,13aR,13bR)-5a,5b,10,10,13b-pentamethyl-3-oxo-1,2,3,3a,4,5,5a,5b,6,7,8,9,10,11,11a,13,13a,13b-octadecahydro-7aH-cyclopenta[a]chrysene-7a-carboxylate |
| Description | Oleanolic acid derivative 2 is an oleanolic acid derivative, which is a novel triterpenoid-steroid hybrid molecule. |
|---|---|
| Related Catalog | |
| In Vitro | Oleanolic acid suppress transcription or translation of inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) genes. HY-18003 is extracted from the reference, compound 12a. For the transformation of olefin 9a (HY-18002) into ketone 12a (HY-18003), the obvious ozonolysis cannot be used because ozone also reacts with the C-12 olefin of oleanane triterpenoids to give the 12R,13R-epoxide, 12-ketone,9 and 12R-hydroxy lactone.[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 498.6±45.0 °C at 760 mmHg |
| Molecular Formula | C28H42O3 |
| Molecular Weight | 426.631 |
| Flash Point | 210.2±28.8 °C |
| Exact Mass | 426.313385 |
| LogP | 7.38 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.544 |
| Storage condition | 2-8℃ |