1505484-82-1
| Name | Nemorexant |
|---|---|
| Synonyms |
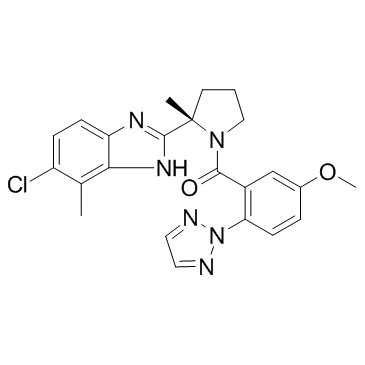
[(2S)-2-(5-Chloro-4-methyl-1H-benzimidazol-2-yl)-2-methyl-1-pyrrolidinyl][5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone
LMQ24G57E9 |
| Description | Nemorexant is a potent orexin receptor antagonist extracted from patent WO2015083094A1, compound example 7, has IC50s of 2 nM and 3 nM for Ox1 receptor and Ox2 receptor, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2 nM (Ox1 receptor ), 3 nM (Ox2 receptor)[1] |
| In Vitro | Nemorexant (Compound example 7) is a orexin receptor antagonist with IC50s of 2 nM and 3 nM for Ox1 receptor and Ox2 receptor, respectively[1]. |
| Cell Assay | Chinese hamster ovary (CHO) cells expressing the human orexin-1 receptor and the human orexin-2 receptor, respectively, are grown in culture medium (Ham F-12 with L-Glutamine) containing 300 μg/mL G418, 100 U/mL Penicillin, 100 μg/mL Streptomycin and 10 % heat inactivated fetal calf serum (FCS). The cells are seeded at 20,000 cells / well into 384-well black clear bottom sterile plates. The seeded plates are incubated overnight at 37°C in 5% CO2. Human orexin-A as an agonist is prepared as 1 mM stock solution in MeOH: water (1 :1), diluted in HBSS containing 0.1 % bovine serum albumin (BSA), NaHCO3: 0.375g/L and 20 mM HEPES for use in the assay at a final concentration of 3 nM. Antagonists are prepared as 10 mM stock solution in DMSO, then diluted in 384-well plates using DMSO followed by a transfer of the dilutions into in HBSS containing 0.1 % bovine serum albumin (BSA), NaHCO3: 0.375g/L and 20 mM HEPES. On the day of the assay, 50 μL of staining buffer (HBSS containing 1 % FCS, 20 mM HEPES, NaHCO3: 0.375g/L, 5 mM probenecid and 3 μM of the fluorescent calcium indicator fluo-4 AM (1 mM stock solution in DMSO, containing 10% pluronic) is added to each well. The 384-well cell-plates are incubated for 50 min at 37°C in 5% CO2 followed by equilibration at RT for 30 min before measurement. Within the Fluorescent Imaging Plate Reader, antagonists are added to the plate in a volume of 10 μL/well, incubated for 120 min and finally 10 μL/well of agonist is added. Fluorescence is measured for each well at 1 second intervals, and the height of each fluorescence peak is compared to the height of the fluorescence peak induced by an approximate EC70 (for example 5 nM) of orexin-A with vehicle in place of antagonist. The IC50 value is determined[1]. |
| References |
[1]. BOSS, Christoph, et al. USE OF BENZIMIDAZOLE-PROLINE DERIVATIVES. WO2015083094A1. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 747.6±70.0 °C at 760 mmHg |
| Molecular Formula | C23H23ClN6O2 |
| Molecular Weight | 450.921 |
| Flash Point | 405.9±35.7 °C |
| Exact Mass | 450.157104 |
| LogP | 3.38 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.704 |
| Storage condition | 2-8℃ |