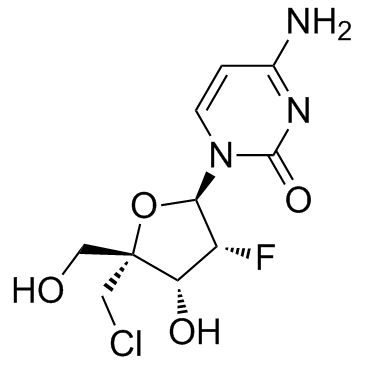
1445379-92-9
| Name | ALS-8112 |
|---|---|
| Synonyms |
7795987MKM
4-Amino-1-[5-chloro-2,5-dideoxy-2-fluoro-4-(hydroxymethyl)-α-L-lyxofuranosyl]-2(1H)-pyrimidinone 4'-CHLOROMETHYL-2'-DEOXY-2'-FLUOROCYTIDINE |
| Description | ALS-8112 is a potent and selective respiratory syncytial virus (RSV) polymerase inhibitor. The 5'-triphosphate form of ALS-8112 inhibits RSV polymerase with an IC50 of 0.02 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.02 μM (RSV)[1] |
| In Vitro | The 5'-triphosphate form of ALS-8112 (ALS-8112-TP) is the active form of the drug and selectively inhibits RSV polymerase through chain termination of RNA synthesis[2]. ALS-008112 enters various types of epithelial cells in the respiratory tract and is subsequently phosphorylated to form an intracellular nucleoside triphosphate with a half-life of approximately 29 hours. The nucleoside triphosphate analogue inhibits RSV replication by means of chain termination[3]. ALS-8112 is a pan-strain inhibitor of RSV replication in vitro. The RNA transcription activity of the RSV–RNP complex is dose-proportionally inhibited by ALS-8112-TP with an IC50 of 0.020 ± 0.008 μM[4]. |
| Cell Assay | ALS-8112 and its prodrug ALS-8176 are stored at 4°C in dimethyl sulfoxide (DMSO), and diluted in water. HEp-2 cells per well are plated in a 96-well plate. Each compound is serially diluted (1:3) up to 9 distinct concentrations. Cells are pre-incubated with compounds for 24 hours at 37°C in a 5% CO2 atmosphere. After 24 hours of pre-incubation with compounds, RSV A2, Long, or B1 at a multiplicity of infection (MOI) of 0.5 is added to the cells, except for the background controls. The plate is then incubated for additional 4 days in the same conditions and at the end of the incubation 50 μL the supernatant from each well of the plate is collected[4]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 532.8±60.0 °C at 760 mmHg |
| Molecular Formula | C10H13ClFN3O4 |
| Molecular Weight | 293.679 |
| Flash Point | 276.0±32.9 °C |
| Exact Mass | 293.057861 |
| LogP | -0.42 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Storage condition | 2-8℃ |