1219805-53-4
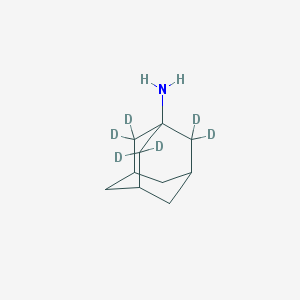
| Name | 1-Aminoadamantane-2,2,2′,2′,2″,2″-d6 |
|---|
| Description | Amantadine-d6 is the deuterium labeled Amantadine[1]. Amantadine (1-Adamantanamine) is an orally avtive and potent antiviral agent with activity against influenza A viruses. Amantadine inhibits several ion channels such as NMDA and M2, and also inhibits Coronavirus ion channels. Amantadine also has anti-orthopoxvirus and anticancer activity. Amantadine can be used for Parkinson's disease, postoperative cognitive dysfunction (POCD) and COVID-19 research[2][3][4][5][6][7]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
[5]. Fink K, et al. Amantadine Inhibits SARS-CoV-2 In Vitro. Viruses. 2021 Mar 2413(4):539. |
| Molecular Formula | C10H17N |
|---|---|
| Molecular Weight | 157.29 |
| Exact Mass | 157.174 |