41078-02-8
| Name | enprofylline |
|---|---|
| Synonyms |
3,7-dihydro-3-propyl-1H-purine-2,6-dione
enprophylline 3-propylxanthine Enprofilina [INN-Spanish] EINECS 255-201-8 3-propyl-7H-purine-2,6-dione Enprofilina Nilyph Oxeze Enprofyllinum [INN-Latin] Enprofyllinum 3-propylxanthine (PX) MFCD00043205 3-n-propylxanthine |
| Description | Enprofylline acts as a selective and competitive A2B receptor antagonist with the Ki of 7 μM. Enprofylline also acts as a phosphodiesterase inhibitor. Enprofylline can be used for the research of asthma, chronic obstructive pulmonary disease[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
A2B receptor:7 μM (Ki) |
| In Vitro | Enprofylline (300 μM) completely blocks the release of IL-8 by N-ethylcarboxamidoadenosine (NECA)[1]. Enprofylline (10 μM) inhibits NECA (10 μM) induced proliferation in a concentration-dependent manner[2]. Cell Proliferation Assay[2] Cell Line: Human retinal endothelial cells (HRECs) Concentration: 10 μM Incubation Time: 24, 48, 72 hours Result: NECA (10 μM) induced a time-dependent increase in HREC proliferation as measured by cell counts, achieving approximately 80% of the density of cells exposed to normal growth medium for 3 days. Enprofylline (10 μM) completely blocked the proliferative effect of NECA when added concurrently with the analogue. |
| In Vivo | Enprofylline increases heart rate (HR). Injection of Enprofylline (7.5 and 30 mg/kg) increases HR in male WT mouse from 529±23 to 590±20 and 562±20 after the low and high dose, respectively[3]. A high dose of Enprofylline (30 mg/kg) also decreases temperature compared with saline injection in female (but not in males) WT mice, but a low dose (7.5 mg/kg) has little effect on mouse temperature[3]. Animal Model: A1RKO mice (were cross-bred to C57BL/6 mice for six generations) and A2ARKO mice (were backcrossed to C57BL/6 mice for more than 10 generations)[3] Dosage: 30 mg/kg Administration: Intraperitoneally injected (bolus) at 2-h intervals Result: Increased HR in male WT mouse from 529±23 to 590±20 and 562±20 after the low (7.5 mg/kg) and high dose (30 mg/kg), respectively. |
| References |
| Density | 1.367 g/cm3 |
|---|---|
| Molecular Formula | C8H10N4O2 |
| Molecular Weight | 194.19100 |
| Exact Mass | 194.08000 |
| PSA | 83.54000 |
| Index of Refraction | 1.583 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | 36 |
| HS Code | 2933990090 |
|
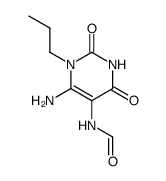
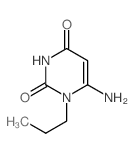
~82% 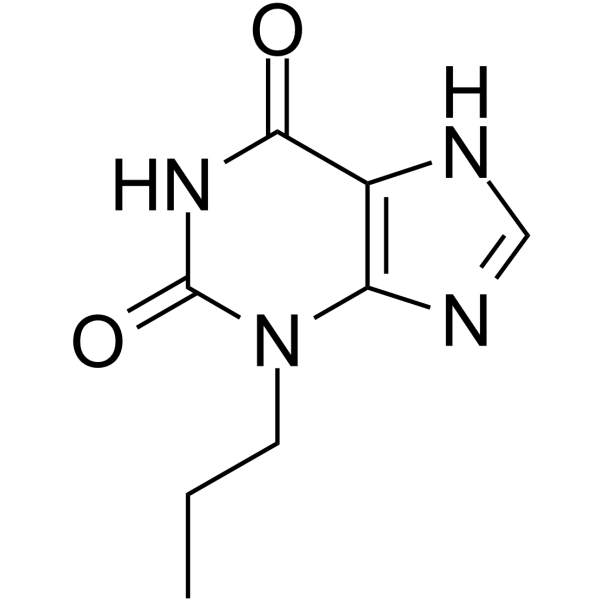
41078-02-8 |
| Literature: Sakai, Ryosuke; Konno, Kayo; Yamamoto, Yasunori; Sanae, Fujiko; Takagi, Kenzo; et al. Journal of Medicinal Chemistry, 1992 , vol. 35, # 22 p. 4039 - 4044 |
|
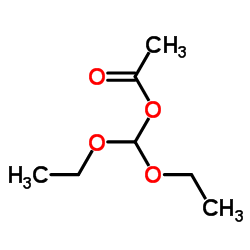
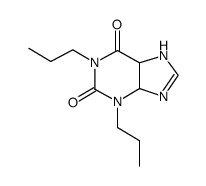
~87% 
41078-02-8 |
| Literature: Cottam, Howard B.; Shih, Hsiencheng; Tehrani, Lida R.; Wasson, D. Bruce; Carson, Dennis A. Journal of Medicinal Chemistry, 1996 , vol. 39, # 1 p. 2 - 9 |
|
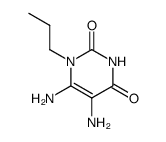
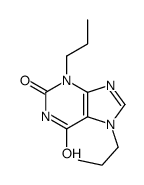
~69% 
41078-02-8 |
| Literature: Aktiebolaget Draco Patent: US4325956 A1, 1982 ; |
|
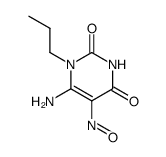
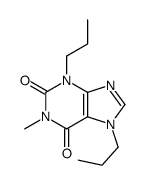
~% 
41078-02-8 |
| Literature: Cottam, Howard B.; Shih, Hsiencheng; Tehrani, Lida R.; Wasson, D. Bruce; Carson, Dennis A. Journal of Medicinal Chemistry, 1996 , vol. 39, # 1 p. 2 - 9 |
|
~% 
41078-02-8 |
| Literature: Cottam, Howard B.; Shih, Hsiencheng; Tehrani, Lida R.; Wasson, D. Bruce; Carson, Dennis A. Journal of Medicinal Chemistry, 1996 , vol. 39, # 1 p. 2 - 9 |
| Precursor 4 | |
|---|---|
| DownStream 5 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |