93957-55-2
| Name | Fluvastatin Sodium Salt Hydrate |
|---|---|
| Synonyms |
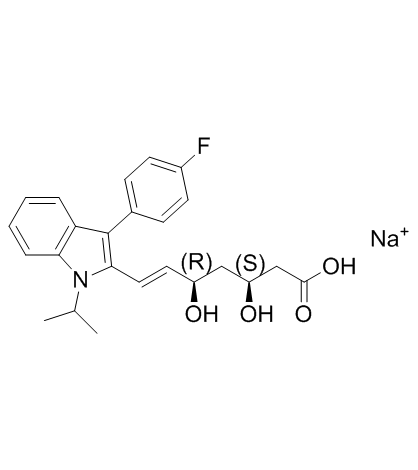
6-Heptenoic acid, 7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-, sodium salt, (3R,5S,6E)- (1:1)
Natrium-(3R,5S,6E)-7-[3-(4-fluorphenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoat sodium (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate Fluvastatin sodium salt Lipaxan MFCD00929076 XU 62-320 fluindostatin (3R,5S,6E)-7-[3-(4-fluorophényl)-1-(1-méthyléthyl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-énoate de sodium Fluvastatin sodium sodium (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate Sodium (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoate Sodium (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate Fluvastatinsodium Fluvastatin (sodium) |
| Description | Fluvastatin sodium is a competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase (HMGCR), used to treat hypercholesterolemia and to prevent cardiovascular disease.Target: HMGCR Fluvastatin is a competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase (HMGCR), the enzyme that catalyzes the conversion of HMG-CoA to mevalonic acid, the rate-limiting step in cholesterol biosynthesis. Human hepatocellular carcinoma cell (HCC) studies indicate that Fluvastatin induces G2/M phase arrest. In the presence of Fluvastatin, HCC cells show a decrease of Bcl-2 and procaspase-9 expression, and an increase in Bax, cleaved caspase-3, and cytochrome c. Fluvastatin is antilipemic and is used to reduce plasma cholesterol levels and prevent cardiovascular disease. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 681.8ºC at 760 mmHg |
|---|---|
| Melting Point | 194-197ºC |
| Molecular Formula | C24H26FNNaO4+ |
| Molecular Weight | 434.46 |
| PSA | 85.52000 |
| LogP | 3.29340 |
| Storage condition | Desiccate at -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |