25425-12-1
| Name | (-)-citreoviridin |
|---|---|
| Synonyms |
Citreoviridin
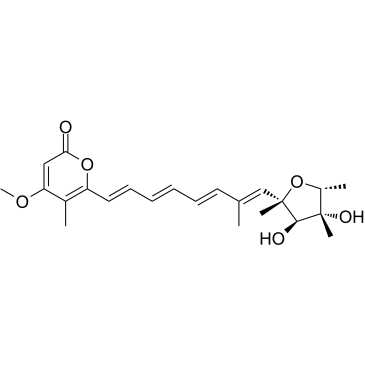
2,5-Anhydro-1,6-dideoxy-2-[(1E,3E,5E,7E)-8-(4-methoxy-5-methyl-2-oxo-2H-pyran-6-yl)-2-methyl-1,3,5,7-octatetraen-1-yl]-4-C-methyl-D-iditol CITREOVIRIDIN BIOCHEMICA Citreoviridin A |
| Description | Citreoviridin, a toxin from Penicillium citreoviride NRRL 2579, inhibits brain synaptosomal Na+/K+-ATPase whereas in microsomes, both Na+/K+-ATPase and Mg2+-ATPase activities are significantly stimulated in a dose-dependent manner[1]. Citreoviridin inhibits cell proliferation and enhances apoptosis of human umbilical vein endothelial cells[2]. |
|---|---|
| Related Catalog | |
| Target |
Na+/K+-ATPase[1] Apoptosis[2] |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 585.1±50.0 °C at 760 mmHg |
| Melting Point | 107-111℃ |
| Molecular Formula | C23H30O6 |
| Molecular Weight | 402.481 |
| Flash Point | 197.7±23.6 °C |
| Exact Mass | 402.204254 |
| PSA | 89.13000 |
| LogP | 3.04 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.569 |
| Storage condition | 2-8°C |
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H311-H315-H319-H331-H335-H361 |
| Precautionary Statements | P261-P264-P280-P301 + P310-P305 + P351 + P338-P311 |
| Hazard Codes | T |
| Risk Phrases | 63-23/24/25-36/37/38 |
| Safety Phrases | 22-26-36/37/39-45 |
| RIDADR | UN 3172 |
| RTECS | UQ1235000 |
| Packaging Group | II |
| HS Code | 29322090 |