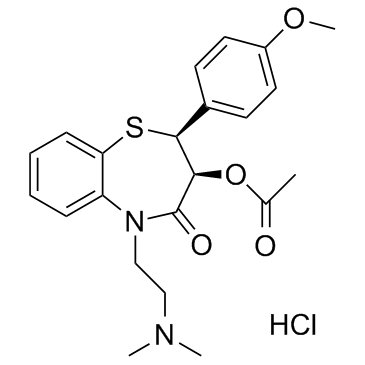
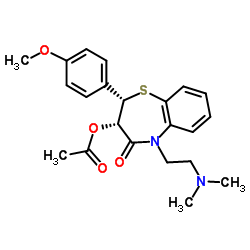
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DL0310000
-
CHEMICAL NAME :
-
1,5-Benzothiazepin-4(5H)-one, 2,3-dihydro-3-(acetyloxy)-5-(2-(dimethylamino)ethyl)- 2-(4- methoxyphenyl)-, monohydrochloride, cis-(+)-
-
CAS REGISTRY NUMBER :
-
33286-22-5
-
LAST UPDATED :
-
199607
-
DATA ITEMS CITED :
-
37
-
MOLECULAR FORMULA :
-
C22-H26-N2-O4-S.Cl-H
-
MOLECULAR WEIGHT :
-
451.02
-
WISWESSER LINE NOTATION :
-
T67 GNV KS&TJ G2N1&1 IOV1 JR DO1 &GH
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
21 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - cardiomyopathy including infarction Cardiac - pulse rate Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
PGMJAO Postgraduate Medical Journal. (Blackwell Scientific Pub. Ltd., POB 88, Oxford, UK) V.1- 1925- Volume(issue)/page/year: 69,474,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
3429 ug/kg/2D-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 341,967,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
8400 ug/kg
-
TOXIC EFFECTS :
-
Cardiac - pulse rate Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
AEMED3 Annals of Emergency Medicine. (American College of Emergency Physicians, 1125 Executive Circle, Irving, TX 75038) Volume(issue)/page/year: 22,196,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
18 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - cardiomyopathy including infarction Cardiac - pulse rate Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
JTCTDW Journal of Toxicology, Clinical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.19- 1982- Volume(issue)/page/year: 29,45,1991
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
120 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - cardiomyopathy including infarction
-
REFERENCE :
-
HETOEA Human & Experimental Toxicology. (Macmillan Press Ltd., Brunel Road, Houndmills, Basingstoke, Hampshire, RG21 2XS, UK) V.9- 1990- Volume(issue)/page/year: 13,161,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
19 mg/kg
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
PGMJAO Postgraduate Medical Journal. (Blackwell Scientific Pub. Ltd., POB 88, Oxford, UK) V.1- 1925- Volume(issue)/page/year: 64,467,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
36 mg/kg/13D-I
-
TOXIC EFFECTS :
-
Liver - hepatitis, fibrous (cirrhosis, post-necrotic scarring)
-
REFERENCE :
-
GASTAB Gastroenterology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1943- Volume(issue)/page/year: 88,1260,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
18 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Vascular - BP lowering not characterized in autonomic section Skin and Appendages - sweating
-
REFERENCE :
-
JTCTDW Journal of Toxicology, Clinical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.19- 1982- Volume(issue)/page/year: 29,45,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
7200 ug/kg/2D-I
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - toxic psychosis
-
REFERENCE :
-
JRSMD9 Journal of the Royal Society of Medicine. (Oxford Univ. Press, Walton St., Oxford OX2 6DP, UK) V.71- 1978- Volume(issue)/page/year: 81,296,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
7200 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - toxic psychosis
-
REFERENCE :
-
JRSMD9 Journal of the Royal Society of Medicine. (Oxford Univ. Press, Walton St., Oxford OX2 6DP, UK) V.71- 1978- Volume(issue)/page/year: 81,296,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
560 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,467,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
520 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,467,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
38 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Behavioral - tetany
-
REFERENCE :
-
JMGZAI Japan Medical Gazette. (Tokyo, Japan) V.1-18(?), 1964-81. Discontinued. Volume(issue)/page/year: 11(1),12,1974
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
508 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 33,2192,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
177 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 29,820,1986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
260 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,467,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
58 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,467,1972
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
40 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 21,4843,1987 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
45500 mg/kg/26W-C
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases Related to Chronic Data - death
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 8,757,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1638 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Endocrine - changes in adrenal weight Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 21,4851,1987 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1200 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
700 mg/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
480 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
OFAJAE Okajimas Folia Anatomica Japonica. (Keio Univ., School of Medicine, Dept. of Anatomy, 35 Shinano-machi, Shinjuku-ku, Tokyo 160, Japan) V.14- 1936- Volume(issue)/page/year: 52,103,1975
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
80 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OFAJAE Okajimas Folia Anatomica Japonica. (Keio Univ., School of Medicine, Dept. of Anatomy, 35 Shinano-machi, Shinjuku-ku, Tokyo 160, Japan) V.14- 1936- Volume(issue)/page/year: 52,103,1975
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
130 mg/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 21,4869,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
378 mg/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating female 1-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 21,4857,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - sex ratio
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 8,3401,1974
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
12500 ug/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
OFAJAE Okajimas Folia Anatomica Japonica. (Keio Univ., School of Medicine, Dept. of Anatomy, 35 Shinano-machi, Shinjuku-ku, Tokyo 160, Japan) V.14- 1936- Volume(issue)/page/year: 52,103,1975
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OFAJAE Okajimas Folia Anatomica Japonica. (Keio Univ., School of Medicine, Dept. of Anatomy, 35 Shinano-machi, Shinjuku-ku, Tokyo 160, Japan) V.14- 1936- Volume(issue)/page/year: 52,103,1975
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OFAJAE Okajimas Folia Anatomica Japonica. (Keio Univ., School of Medicine, Dept. of Anatomy, 35 Shinano-machi, Shinjuku-ku, Tokyo 160, Japan) V.14- 1936- Volume(issue)/page/year: 52,103,1975
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
OFAJAE Okajimas Folia Anatomica Japonica. (Keio Univ., School of Medicine, Dept. of Anatomy, 35 Shinano-machi, Shinjuku-ku, Tokyo 160, Japan) V.14- 1936- Volume(issue)/page/year: 52,103,1975
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
125 mg/kg
-
SEX/DURATION :
-
female 7-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OFAJAE Okajimas Folia Anatomica Japonica. (Keio Univ., School of Medicine, Dept. of Anatomy, 35 Shinano-machi, Shinjuku-ku, Tokyo 160, Japan) V.14- 1936- Volume(issue)/page/year: 52,103,1975
|