489-33-8
| Name | 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-8-methoxychromen-4-one |
|---|---|
| Synonyms |
Limocitrin
3,5,7,4'-tetrahydroxy-8,3'-dimethoxyflavone 3,5,7,4'-Tetrahydroxy-3',8-dimethoxy-flavon 8,3'-dimethoxy gossypetin gossypetin 8,3'-dimethyl ether |
| Description | Limocitrin is a natural product that can be isolated from the buds of P. acerifolia and P. orientalis. Limocitrin suppresses estradiol-dependent proliferation of MCF7 cells weakly but estradiol-induced AlkP (alkaline phosphatase) expression only marginally[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.591g/cm3 |
|---|---|
| Boiling Point | 621.2ºC at 760 mmHg |
| Molecular Formula | C17H14O8 |
| Molecular Weight | 346.28800 |
| Flash Point | 231.4ºC |
| Exact Mass | 346.06900 |
| PSA | 129.59000 |
| LogP | 2.29960 |
| Vapour Pressure | 5.03E-16mmHg at 25°C |
| Index of Refraction | 1.707 |
|
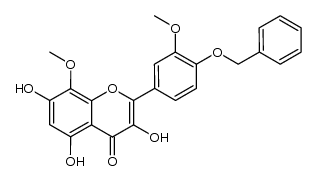
~85% 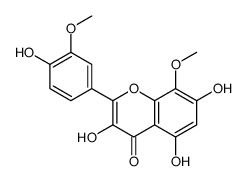
489-33-8 |
| Literature: Horie; Tsukayama; Kawamura; Seno; Yamamoto Bulletin of the Chemical Society of Japan, 1988 , vol. 61, # 2 p. 441-447 |
|
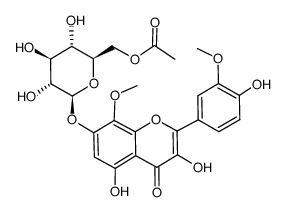
~% 
489-33-8 |
| Literature: Horie; Tsukayama; Kawamura; Seno; Yamamoto Bulletin of the Chemical Society of Japan, 1988 , vol. 61, # 2 p. 441-447 |
|
~% 
489-33-8 |
| Literature: Horie; Tsukayama; Kawamura; Seno; Yamamoto Bulletin of the Chemical Society of Japan, 1988 , vol. 61, # 2 p. 441-447 |
|
~% 
489-33-8 |
| Literature: Yuldashev Chemistry of Natural Compounds, 2001 , vol. 37, # 3 p. 288 - 289 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |