523-66-0
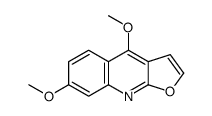
| Name | 4,7-dimethoxyfuro[2,3-b]quinoline |
|---|---|
| Synonyms |
Furo(2,3-b)quinoline,4,7-dimethoxy
Evolitrine Evolitrin |
| Description | Evolitrine (7-Methoxydictamnine; Evolitrin) is isolated from Acronychia pedunculata and show anti-inflammatory and antifeedant activities[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.261g/cm3 |
|---|---|
| Boiling Point | 381ºC at 760mmHg |
| Melting Point | 115℃ |
| Molecular Formula | C13H11NO3 |
| Molecular Weight | 229.23100 |
| Flash Point | 184.2ºC |
| Exact Mass | 229.07400 |
| PSA | 44.49000 |
| LogP | 2.99820 |
| Index of Refraction | 1.642 |
| Storage condition | 2-8℃ |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2934999090 |
|
~% 
523-66-0 |
| Literature: Bhoga, Umadevi; Mali; Adapa, Srinivas R. Tetrahedron Letters, 2004 , vol. 45, # 51 p. 9483 - 9485 |
|
~% 
523-66-0 |
| Literature: Bhoga, Umadevi; Mali; Adapa, Srinivas R. Tetrahedron Letters, 2004 , vol. 45, # 51 p. 9483 - 9485 |
|
~% 
523-66-0 |
| Literature: Cooke; Haynes Australian Journal of Chemistry, 1958 , vol. 11, p. 225,228 |
|
~% 
523-66-0 |
| Literature: Bhoga, Umadevi; Mali; Adapa, Srinivas R. Tetrahedron Letters, 2004 , vol. 45, # 51 p. 9483 - 9485 |
|
~% 
523-66-0 |
| Literature: Bhoga, Umadevi; Mali; Adapa, Srinivas R. Tetrahedron Letters, 2004 , vol. 45, # 51 p. 9483 - 9485 |
|
~% 
523-66-0 |
| Literature: Sato; Ohta Bulletin of the Chemical Society of Japan, 1958 , vol. 31, p. 157,158 |
|
~% 
523-66-0 |
| Literature: Sato; Ohta Bulletin of the Chemical Society of Japan, 1958 , vol. 31, p. 157,158 |
|
~% 
523-66-0 |
| Literature: Sato; Ohta Bulletin of the Chemical Society of Japan, 1958 , vol. 31, p. 157,158 |
|
~% 
523-66-0 |
| Literature: Johns,S.R. et al. Australian Journal of Chemistry, 1968 , vol. 21, p. 1897 - 1901 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |