869886-67-9
| Name | N-[(1S)-1-(3-chlorophenyl)-2-hydroxyethyl]-4-[5-chloro-2-(propan-2-ylamino)pyridin-4-yl]-1H-pyrrole-2-carboxamide |
|---|---|
| Synonyms |
4-[5-Chloro-2-(isopropylamino)-4-pyridinyl]-N-[(1S)-1-(3-chlorophenyl)-2-hydroxyethyl]-1H-pyrrole-2-carboxamide
4-(5-chloro-2-isopropylaminopyridin-4-yl)-1H-pyrrole-2-carboxylic acid [1-(3-chlorophenyl)-2-hydroxyethyl]amide ulixertinib VRT752271 BVD-523 |
| Description | Ulixertinib (VRT752271) is a reversible, ATP-competitive inhibitor of ERK1 and ERK2 kinases, with IC50 of <0.3 nM against ERK2. |
|---|---|
| Related Catalog | |
| Target |
ERK2:0.3 nM (IC50, at KM ATP (60 μM)) ERK1 |
| In Vitro | Ulixertinib (VRT752271) is a reversible, ATP-competitive inhibitor of ERK1 and ERK2 kinases, with IC50 of <0.3 nM against ERK2[2]. |
| In Vivo | In the pharmacokinetic study, the sensitivity and specificity of the assay are found to be sufficient for accurately characterizing the plasma pharmacokinetics of Ulixertinib in Balb/C mice[1]. |
| Kinase Assay | The MEK autophosphorylation assay is performed using the ADP Glo Kinase Assay kit. Activated MEK protein is expressed and purified in-house. Enzyme and substrate solutions are prepared in assay buffer consisting of 50 mM Tris (pH 7.5), 10 mM MgCl2, 0.1 mM EGTA, 10 mM DTT and 0.01 % Tween 20. 6 nM MEK protein is prepared in assay buffer and 2 µL is dispensed into each well of a 384-well white small volume medium bind plate containing test and reference control compounds. Compound plates are dosed with a 12 point dose response curve from 10 µM down to 0.0625 nM in order to calculate compound IC50s, with a total DMSO concentration in the assay of 1%. Following a 15 minute pre-incubation of enzyme and compound at room temperature, 2 µL of a 20 µM Ultra Pure ATP solution in assay buffer is added to the wells, and the reaction is allowed to progress for 90 minutes at room temperature before the addition of 4 µL ADP Glo reagent R1 to quench the reaction. The plate is incubated at room temperature for 45 minutes before the addition of 8 µL Kinase Detection Reagent, and then the luminescence signal is allowed to equilibrate for 60 minutes before the plates are read on a Pherastar plate reader. |
| Cell Assay | A375 cells are cultured in cell media composed of DMEM, 10% (v/v) Foetal Calf Serum and 1% (v/v) L-Glutamine. After harvesting, cells are dispensed into black, 384-well Costar plates to give 200 cells per well in a total volume of 40 µL cell media, and are incubated overnight at 37°C, 90% relative humidity and 5% CO2 in a rotating incubator. Test compounds and reference controls are dosed directly into the cell plates, into the inner 308 wells. The cells are dosed over a 12 point range from 30 µM down to 0.03 nM in order to calculate compound IC50s, with a total DMSO concentration in the assay of 0.3%. The cell plates are then incubated for 72 hours at 37°C. Cells are fixed and stained by the addition of 20 µL 12% formaldehyde in PBS/A (4% final concentration) and 1:2000 dilution of Hoechst 33342, with a 30 minute room temperature incubation, and then washed with PBS/A. A cell count is performed on the stained cell plates using a Cellomics ArrayScanTM VTI imaging platform. A Day 0 cell plate is also fixed, stained and read to generate a cell count baseline for determining compound cytotoxic effects as well as anti-proliferative effects. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 682.8±55.0 °C at 760 mmHg |
| Molecular Formula | C21H22Cl2N4O2 |
| Molecular Weight | 433.331 |
| Flash Point | 366.8±31.5 °C |
| Exact Mass | 432.111969 |
| PSA | 90.04000 |
| LogP | 5.16 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.650 |
| Storage condition | -20℃ |
|
~62% 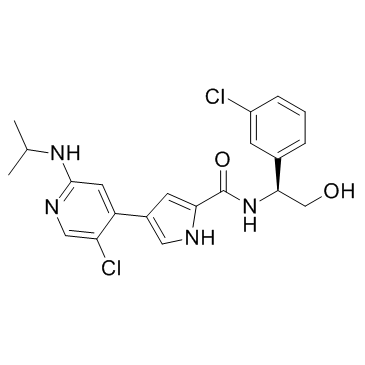
869886-67-9 |
| Literature: VERTEX PHARMACEUTICALS, INCORPORATED Patent: WO2005/113541 A1, 2005 ; Location in patent: Paragraph 0089 ; WO 2005/113541 A1 |
|
~% 
869886-67-9 |
| Literature: VERTEX PHARMACEUTICALS, INCORPORATED Patent: WO2005/113541 A1, 2005 ; Location in patent: Paragraph 0090 ; WO 2005/113541 A1 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |