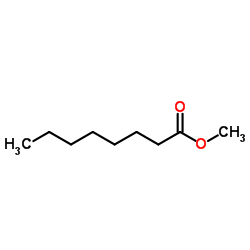
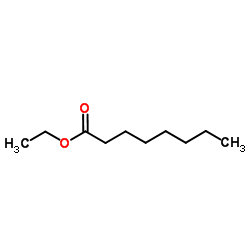
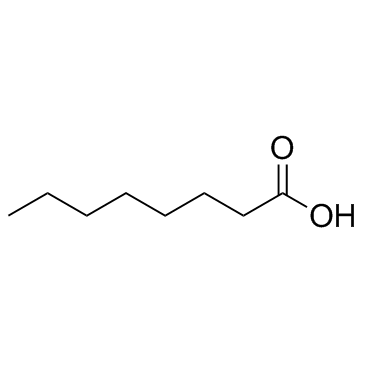
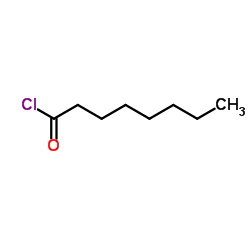
78510-02-8
| Name | 1-octanol-1,1-d2 |
|---|---|
| Synonyms |
1-octanol-1-(2)H2
1-[1,1-D2]octanol 1-[1,1-(2)H2]-octanol <1-2H2>-1-octanol (2H2)ethane <1,1-2H2>octanol |
| Description | 1-Octanol-d2 is the deuterium labeled 1-Octanol[1]. 1-Octanol (Octanol), a saturated fatty alcohol, is a T-type calcium channels (T-channels) inhibitor with an IC50 of 4 μM for native T-currents[2]. 1-Octanol is a highly attractive biofuel with diesel-like properties[3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C8H16D2O |
|---|---|
| Molecular Weight | 132.24000 |
| Exact Mass | 132.14800 |
| PSA | 20.23000 |
| LogP | 2.33920 |
|
~99% 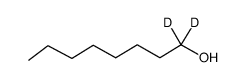
78510-02-8 |
| Literature: Landini, Dario; Maia, Angelamaria; Montanari, Fernando; Rolla, Franco Journal of Organic Chemistry, 1983 , vol. 48, # 21 p. 3774 - 3777 |
|
~97% 
78510-02-8 |
| Literature: Bhar, Palash; Reed, Darwin W.; Covello, Patrick S.; Buist, Peter H. Angewandte Chemie - International Edition, 2012 , vol. 51, # 27 p. 6686 - 6690 |
|
~98% 
78510-02-8 |
| Literature: Chen, Hao; Plettner, Erika Journal of Labelled Compounds and Radiopharmaceuticals, 2012 , vol. 55, # 2 p. 66 - 70 |
|
~57% 
78510-02-8 |
| Literature: Yang, Haishen; Mu, Feng; Wang, Pengfei Journal of Organic Chemistry, 2011 , vol. 76, # 21 p. 8955 - 8961 |
|
~% 
78510-02-8 |
| Literature: Luck et al. Journal of the American Chemical Society, 1959 , vol. 81, p. 2784 |
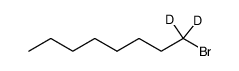
| Precursor 5 | |
|---|---|
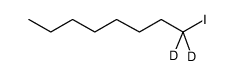
| DownStream 6 | |