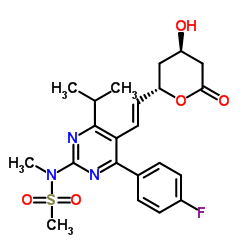
287714-41-4
| Name | rosuvastatin |
|---|---|
| Synonyms |
Reference source: Negwer
volume-3 compound no ROSUVASTATIN-D3 SODIUM SALT 7-[4-(4-Fluorophenyl)-6-(1-methylethyl)-2-(methyl-methylsulfonyl-amino)-pyrimidin-5-yl]-3,5-dihydroxy-hept-6-enoic acid 6-Heptenoic acid, 7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl-d(methylsulfonyl)amino]-5-pyrimidinyl]-3,5-dihydroxy-, sodium salt, (3R,5S,6E)- (1:1) [3H]-Rosuvastatin Rosuvastatin Sodium (3R,5S,6E)-7-{4-(4-fluorophenyl)-6-isopropyl-2-[(H)methyl(methylsulfonyl)amino]-5-pyrimidinyl}-3,5-dihydroxy-6-heptenoate (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-MethylMethanesulfonaMido)-6-(propan-2-yl)pyriMidin-5-yl]-3,5-dihydroxyhept-6-enoic acid MFCD08460959 |
| Description | Rosuvastatin is a competitive inhibitor of HMG-CoA reductase with IC50 of 11 nM. IC50 Value: 11 nM [1]Target: HMG-CoA reductasein vitro: Rosuvastatin is relatively hydrophilic and is highly selective for hepatic cells; its uptake is mediated by the liver-specific organic anion transporter OATP-C. Rosuvastatin is a high-affinity substrate for OATP-C with apparent association constant of 8.5 μM [2]. Rosuvastatin inhibits cholesterol biosynthesis in rat liver isolated hepatocytes with IC50 of 1.12 nM. Rosuvastatin causes approximately 10 times greater increase of mRNA of LDL receptors than pravastatin [1]. Rosuvastatin (100 μM) decreases the extent of U937 adhesion to TNF-α-stimulated HUVEC. Rosuvastatin inhibits the expressions of ICAM-1, MCP-1, IL-8, IL-6, and COX-2 mRNA and protein levels through inhibition of c-Jun N-terminal kinase and nuclear factor-kB in endothelial cells [3].in vivo: Rosuvastatin (3 mg/kg) daily administration for 14 days decreases plasma cholesterol levels by 26% in male beagle dogs with normal cholesterol levels. In cynomolgus monkeys, Rosuvastatin decreases plasma cholesterol levels by 22% [1]. Rosuvastatin (20 mg/kg/day) administration for 2 weeks, significantly reduces very low-density lipoproteins (VLDL) in diabetes mellitus rats induced by Streptozocin [4]. Rosuvastatin shows antiatherothromhotic effects in vivo. Rosuvastatin (1.25 mg/kg) significantly inhibits thrombin-induced transmigration of monocvtes across mesenteric venules via inhibition of the endothelial cell surface expression of P-selectin, and increases the basal rate of nitric oxide in aortic segments by 2-fold times [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.368 g/cm3 |
|---|---|
| Boiling Point | 745.6ºC at 760 mmHg |
| Molecular Formula | C22H28FN3O6S |
| Molecular Weight | 481.54 |
| Flash Point | 404.7ºC |
| PSA | 152.13000 |
| LogP | 2.14780 |
| Vapour Pressure | 2.38E-23mmHg at 25°C |
| Index of Refraction | 1.597 |
| Storage condition | 2-8°C |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
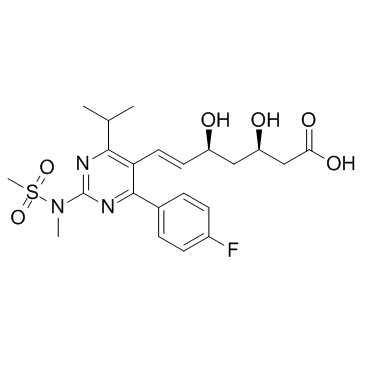
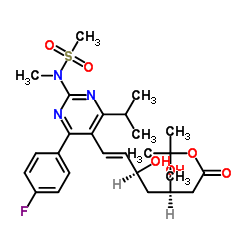
![tert-butyl 2-[6-[(E)-2-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]ethenyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate structure](https://image.chemsrc.com/caspic/488/1007871-85-3.png)
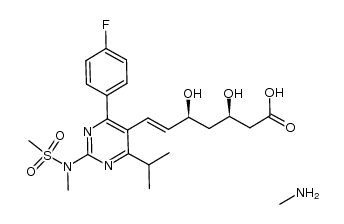
![(+)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl]-(3R,5S)-dihydroxy-(E)-heptenoic acid iso-propylammonium salt structure](https://image.chemsrc.com/caspic/017/852820-97-4.png)
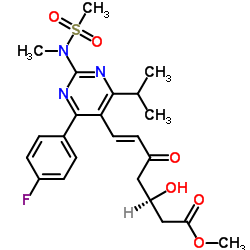
![tert-Butyl 6-[(1E)-2-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetate structure](https://image.chemsrc.com/caspic/152/289042-12-2.png)


![N-[4-(4-Fluorophenyl)-5-formyl-6-(1-methylethyl)-2-pyrimidinyl]-N-methyl-methanesulfonamide structure](https://image.chemsrc.com/caspic/248/147118-37-4.png)