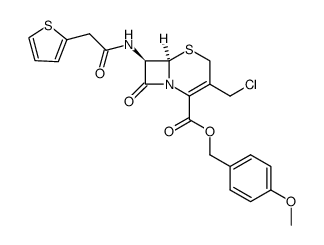
41906-86-9
| Name | nitrocefin |
|---|---|
| Synonyms |
(6R-(3(E),6a,7b))-3-(2-(2,4-Dinitrophenyl)ethenyl)-8-oxo-7-((2-thienylacetyl)amino)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
nitrocefin (6R,7R)-3-[(E)-2-(2,4-Dinitrophenyl)vinyl]-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Description | Nitrocefin is a chromogenic β-lactamase substrate that undergoes distinctive color change from yellow to red as the amide bond in the β-lactam ring is hydrolyzed by β-lactamase. Nitrocefin is used in competitive inhibition studies in developmental work on β-lactamase-resistant antibiotics[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 872.0±65.0 °C at 760 mmHg |
| Melting Point | >99℃ |
| Molecular Formula | C21H16N4O8S2 |
| Molecular Weight | 516.504 |
| Flash Point | 481.2±34.3 °C |
| Exact Mass | 516.040955 |
| PSA | 231.89000 |
| LogP | 1.04 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.749 |
|
~% 
41906-86-9 |
| Literature: Lee, Mijoon; Hesek, Dusan; Mobashery, Shahriar Journal of Organic Chemistry, 2005 , vol. 70, # 1 p. 367 - 369 |
|
~% 
41906-86-9 |
| Literature: Lee, Mijoon; Hesek, Dusan; Mobashery, Shahriar Journal of Organic Chemistry, 2005 , vol. 70, # 1 p. 367 - 369 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |