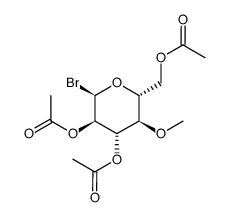
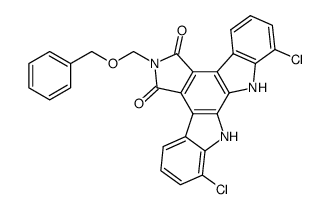
93908-02-2
| Name | rebeccamycin |
|---|---|
| Synonyms |
AmbotzLS-1199
MFCD01718312 |
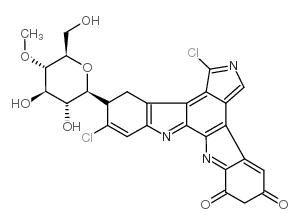
| Description | Rebeccamycin, an antitumor antibiotic, inhibits DNA topoisomerase I. Rebeccamycin appears to exert its primary antineoplastic effect by poisoning topoisomerase I and has negligible effect on protein kinase C and topoisomerase II[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I Traditional Cytotoxic Agents |
| In Vitro | Rebeccamycin is an antitumor antibiotic produced by the actinomycete Saccharotrix aerocolonigenes with activity against several human tumor cell lines, including A549 (lung adenocarcinoma), HCT-116 (colon carcinoma), and KB (nasopharyngeal carcinoma) [1]. Rebeccamycin shows antibacterial activity against several Gram-positive bacteria, including Staphylococcus aureus and Streptococcus faecalis[3]. |
| In Vivo | Rebeccamycin (2-256 mg/kg; i.p.; daily for 9 days) prolongs survival in B16 melanoma, L1210 leukemia[2]. Animal Model: CDF1 mice (B16 melanoma, L1210 leukemia)[2] Dosage: 2, 4, 8, 16, 32, 64, 128, 256 mg/kg Administration: I.p.; daily for 9 days Result: Prolongation of survival of the mice at dose levels ranging from 8 to 256 mg/kg. |
| References |
| Density | 1.87g/cm3 |
|---|---|
| Molecular Formula | C27H21Cl2N3O7 |
| Molecular Weight | 570.37800 |
| Exact Mass | 569.07600 |
| PSA | 150.37000 |
| Index of Refraction | 1.844 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
93908-02-2 |
| Literature: Kaneko; Wong; Okamoto; Clardy Tetrahedron Letters, 1985 , vol. 26, # 34 p. 4015 - 4018 |
|
~% 
93908-02-2 |
| Literature: Kaneko; Wong; Okamoto; Clardy Tetrahedron Letters, 1985 , vol. 26, # 34 p. 4015 - 4018 |
|
~% 
93908-02-2 |
| Literature: Kaneko; Wong; Okamoto; Clardy Tetrahedron Letters, 1985 , vol. 26, # 34 p. 4015 - 4018 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |