Sulbactam-d2 sodium
Modify Date: 2025-08-27 20:41:53
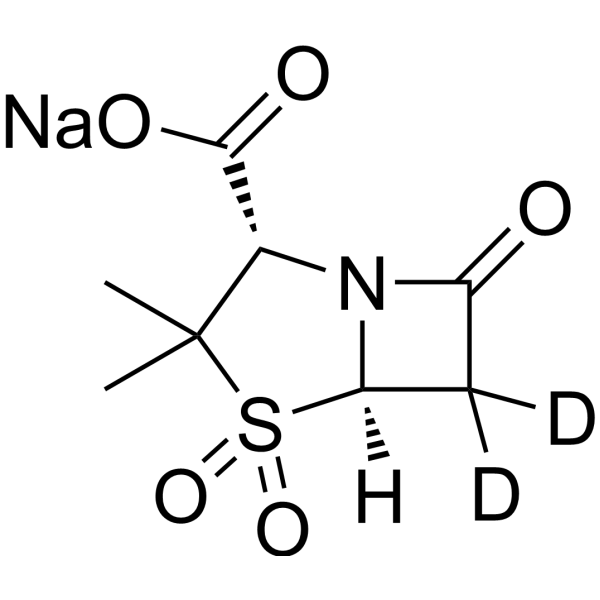
Sulbactam-d2 sodium structure
|
Common Name | Sulbactam-d2 sodium | ||
|---|---|---|---|---|
| CAS Number | 948027-82-5 | Molecular Weight | 257.24 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C8H8D2NNaO5S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Sulbactam-d2 sodiumSulbactam-d2 (sodium) is the deuterium labeled Sulbactam sodium[1]. Sulbactam (CP45899) sodium is a competitive, irreversible beta-lactamase inhibitor. Sulbactam sodium shows antimicrobial activity against multidrug-resistant (MDR) acinetobacter calcoaceticus--Acinetobacter baumannii (Acb) complex[2][3]. |
| Name | Sulbactam-d2 sodium |
|---|
| Description | Sulbactam-d2 (sodium) is the deuterium labeled Sulbactam sodium[1]. Sulbactam (CP45899) sodium is a competitive, irreversible beta-lactamase inhibitor. Sulbactam sodium shows antimicrobial activity against multidrug-resistant (MDR) acinetobacter calcoaceticus--Acinetobacter baumannii (Acb) complex[2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
[2]. Noguchi JK, et al. Sulbactam: a beta-lactamase inhibitor. Clin Pharm. 1988;7(1):37-51. |
| Molecular Formula | C8H8D2NNaO5S |
|---|---|
| Molecular Weight | 257.24 |
| InChIKey | NKZMPZCWBSWAOX-RIUSHSMCSA-M |
| SMILES | CC1(C)C(C(=O)[O-])N2C(=O)CC2S1(=O)=O.[Na+] |