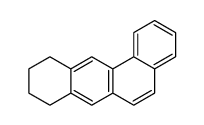
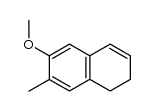
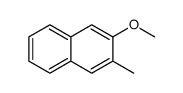
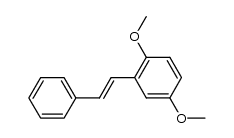
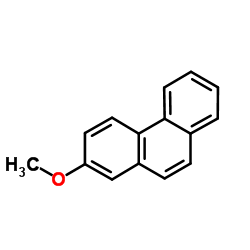
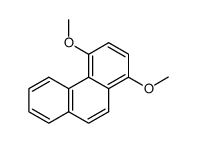
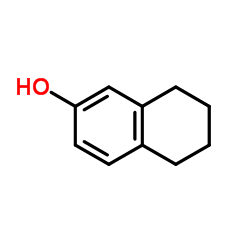
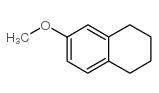
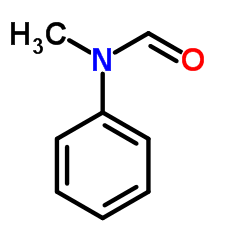
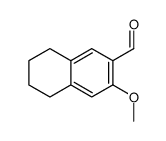
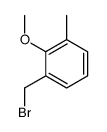
|
~54% |
|
~86% |
|
~79% |
|
~% |
|
~53% |
|
~96% |
|
~58% |
|
~55% |
|
~% |
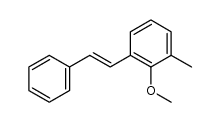

![methyl-(2-methyl-[1]phenanthryl)-ether Structure](https://image.chemsrc.com/caspic/264/122950-62-3.png)